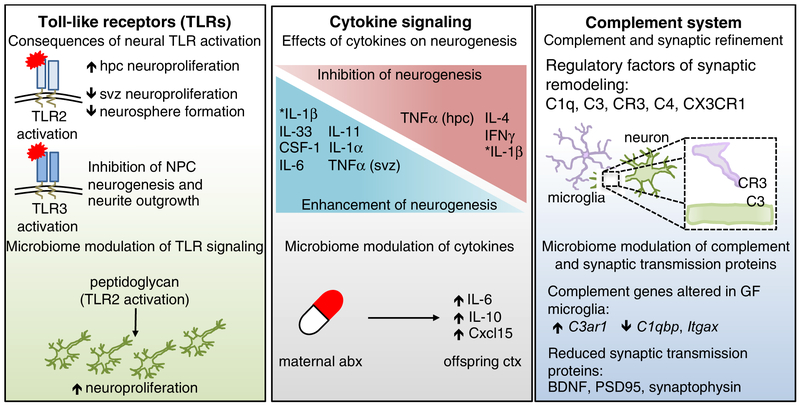
Figure 2. Canonical immune molecules play fundamental roles in normal neurodevelopment.
TLRs are expressed in every major cell type of the brain, and exhibit temporally distinct expression patterns that are critical for normal neurodevelopment. Activation of TLRs can have opposing effects on neuroproliferation and neurogenesis. For example, TLR2 activation increased hippocampal (HPC) neuroproliferation, but reduced neuroproliferation in the subventricular zone (SVZ). Activation of TLR3 resulted in reduced neural progenitor cell neurogenesis and neurite outgrowth, highlighting the differential effects of TLR stimulation in the developing brain. Fetal TLR2 activation by the bacterial cell wall component, peptidoglycan, increased hippocampal neuroproliferation. The developing brain is also highly dependent on a balanced cytokine milieu, as different cytokines can have opposing effects on neurogenesis. Maternal treatment with antibiotics resulted in sustained increases in IL-6, IL-10 and Cxcl15 in the cortex of offspring. Further, cytokines induced by maternal immune activation (MIA), were dependent on the maternal microbiota, such as increased prevalence of segmented filamentous bacteria (SFB), which led to exacerbated behavioral deficits in offspring. The complement system is critical for synaptic editing and refinement of neural circuits. Recognition of neuronal C3 by microglial-expressed C3R is important for this process, as are C1q and C4. Mice lacking the fractalkine receptor, CX3CR1, also exhibit disrupted synaptic editing. GF microglia exhibited altered gene expression of complement-associated genes, such as increased C3ar1 and decreased C1qbp and Itgax. Proteins essential for synaptic transmission, such as BDNF, synaptophysin, and PSD95, are also reduced in microbiota-depleted mice. Many other molecules, such as the aryl hydrocarbon receptor (AhR) and MHCI impact neurodevelopment as well (Bryceson et al., 2005; Glynn et al., 2011; Kimura et al., 2016; Latchney et al., 2013; Zohar et al., 2008). Deletion of AhR reduced cell proliferation and neuronal differentiation in the dentate gyrus (DG). Reduction in surface-expressed MHCI resulted in increased synapse density. Several MHCI receptors are expressed in the brain, including PirB, CD3z, Ly49 and KIR.