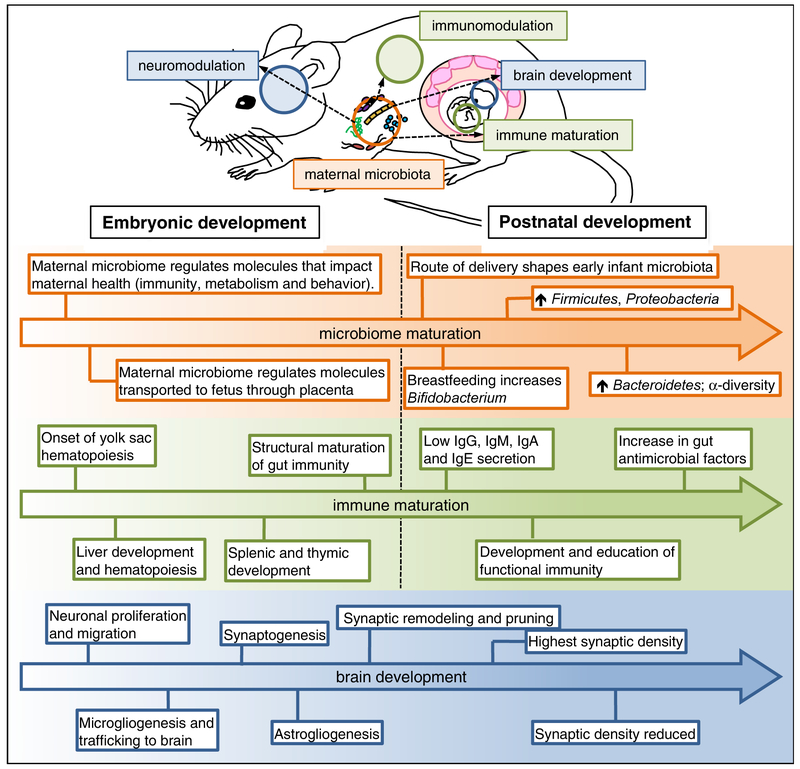
Figure 3. The maternal microbiome influences fetal immune and brain development.
The maternal microbiome produces microbe-associated molecular patterns and secondary metabolites that can impact the developing fetus. Several factors influence the initial acquisition of microbes, including the route of delivery and breast-feeding. The initial infant gut microbiota is dominated by Firmicutes and Proteobacteria, and experiences diet-related shifts, such as the increase in Bifidobacterium following breast-feeding, and Bacteroidetes following a transition to solid foods. Bacterial alpha-diversity increases, eventually reaching complexity similar to the adult. Antimicrobial factors in the gut increase concurrent to the changing infant microbiome. Maturation of the immune system also begins in the fetal period and persists into postnatal development. Hematopoiesis first occurs in the yolk sac and continues in the fetal liver. Lymphoid organs, such as the spleen and thymus, follow, and immune circulating immune cells are also found in the developing fetus. Structural maturation of gut immunity is evident just before birth, although immune function is reduced during this phase. The fetus is capable of producing low levels of immunoglobulins, such as IgG, but relies heavily on maternal immunoglobulins. Following birth, acquisition of immunoglobulins is facilitated through breast-milk. Brain development occurs concurrently over defined stages, starting with the proliferation and migration of neuronal progenitor cells. Shortly after, immature macrophages from the yolk sac migrate to the central nervous system where they mature to resident microglial cells. Shortly after, wiring of the brain circuitry begins with the onset of synaptogenesis. During the late gestational period, astrocytes develop to facilitate the increasing metabolic demand for brain growth. During the late fetal period, and into early postnatal development, synaptic remodeling and pruning occurs, with studies demonstrating a clear role for microglial mediated synaptic editing as well as astrocyte-mediated pruning of the synapse.