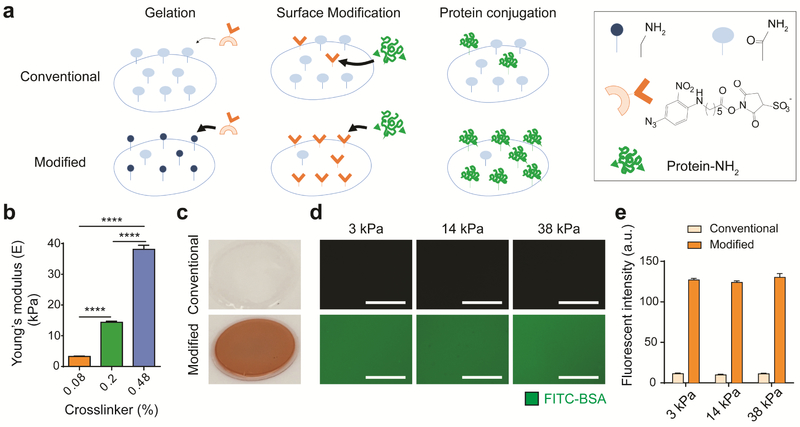
Figure 1. Modified polyacrylamide hydrogel with primary amines significantly enhances protein conjugation efficiency regardless of hydrogel stiffness.
a Schematic of polyacrylamide hydrogel conjugation. On polyacrylamide hydrogels fabricated by the conventional method, only a few amide groups ( ) react with photoactivated nitrophenyl azide groups (
) react with photoactivated nitrophenyl azide groups ( ) from the sulfo-SANPAH crosslinker, leading to low protein conjugation efficiency. When primary amine groups (
) from the sulfo-SANPAH crosslinker, leading to low protein conjugation efficiency. When primary amine groups ( ) are introduced into the polyacrylamide hydrogel surface, photoactivated nitrophenyl azide groups dominantly react with primary amines, thereby enhancing the amount of NHS groups (
) are introduced into the polyacrylamide hydrogel surface, photoactivated nitrophenyl azide groups dominantly react with primary amines, thereby enhancing the amount of NHS groups ( ) from the sulfo-SANPAH crosslinker on the surface for conjugation of amine-containing proteins. b Young’s moduli of three polyacrylamide hydrogel groups determined via atomic force microscopy. Data are mean values; standard error of the mean is given as error bars. (n=18, ****p<0.0001, One-way ANOVA by Tukey’s multiple comparisons test) c Photograph of polyacrylamide hydrogels after sulfo-SANPAH incorporation. The orange color of the modified polyacrylamide hydrogel indicates high incorporation of orange sulfo-SANPAH, whereas the conventional hydrogel remains clear. d Fluorescence image of FITC-BSA incorporation into polyacrylamide hydrogels fabricated with the conventional or modified protocol. With the same exposure time, the hydrogel with primary amine groups more efficiently conjugated FITC-BSA than the hydrogel without primary amine groups. Scale bar: 100 μm. e Quantification of fluorescence of FITC-BSA on conventional or modified polyacrylamide hydrogels of three stiffnesses. Modified polyacrylamide hydrogels with primary amine groups show a 10-fold higher fluorescence intensity than hydrogels without primary amine groups. Data are mean values; error bars are standard error of the mean. (n=3)
) from the sulfo-SANPAH crosslinker on the surface for conjugation of amine-containing proteins. b Young’s moduli of three polyacrylamide hydrogel groups determined via atomic force microscopy. Data are mean values; standard error of the mean is given as error bars. (n=18, ****p<0.0001, One-way ANOVA by Tukey’s multiple comparisons test) c Photograph of polyacrylamide hydrogels after sulfo-SANPAH incorporation. The orange color of the modified polyacrylamide hydrogel indicates high incorporation of orange sulfo-SANPAH, whereas the conventional hydrogel remains clear. d Fluorescence image of FITC-BSA incorporation into polyacrylamide hydrogels fabricated with the conventional or modified protocol. With the same exposure time, the hydrogel with primary amine groups more efficiently conjugated FITC-BSA than the hydrogel without primary amine groups. Scale bar: 100 μm. e Quantification of fluorescence of FITC-BSA on conventional or modified polyacrylamide hydrogels of three stiffnesses. Modified polyacrylamide hydrogels with primary amine groups show a 10-fold higher fluorescence intensity than hydrogels without primary amine groups. Data are mean values; error bars are standard error of the mean. (n=3)