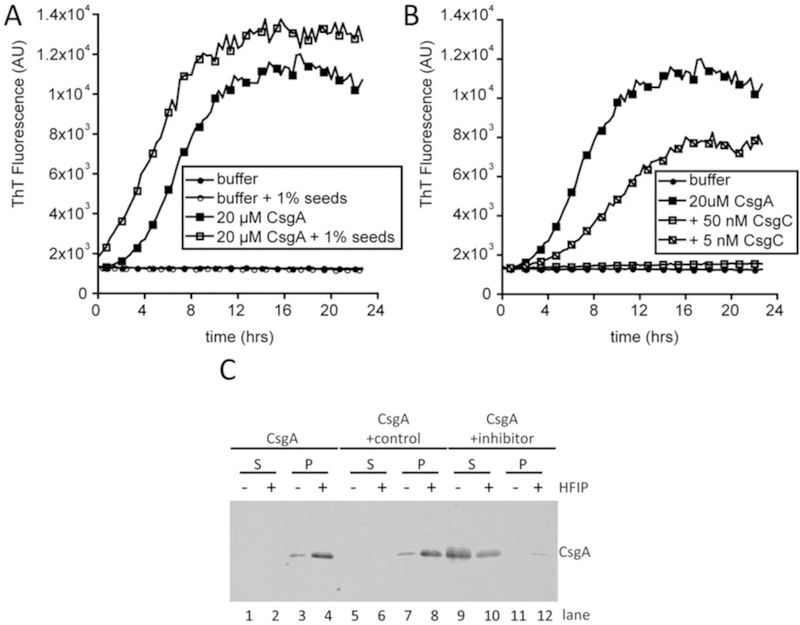
Fig. 4.
CsgA assembles into an amyloid in vitro. (a) Polymerization of CsgA was monitored by ThT fluorescence in a plate reader over 24 h. Buffer alone (closed circles) and with sonicated CsgA fibers (seeds; open circles) do not bind ThT. CsgA alone (closed squares in a and b) rapidly polymerizes and binds ThT after approximately 2 h. The 2-h lag phase is overcome by the addition of CsgA seeds to CsgA monomers (open squares). (b) CsgA amyloid assembly can be inhibited by protein inhibitors such as CsgC. CsgC was added to purified CsgA at sub-stoichiometric molar ratios. The addition of 50 nM CsgC to 20 μM (1:400 molar ratio) completely inhibits CsgA amyloid assembly (open squares) whereas the addition of 5 μM CsgC (1:4000 molar ratio) extends the lag phase and rate of CsgA amyloid assembly (slashed squares). (c) Soluble (S) and polymerized (P) CsgA can be distinguished using a gel solubility assay. CsgA was incubated alone (lanes 1–4), with a control protein that does not affect amyloid formation (lanes 5–8) or with an inhibitory protein (lanes 9–12) prior to separating the soluble and polymerized species