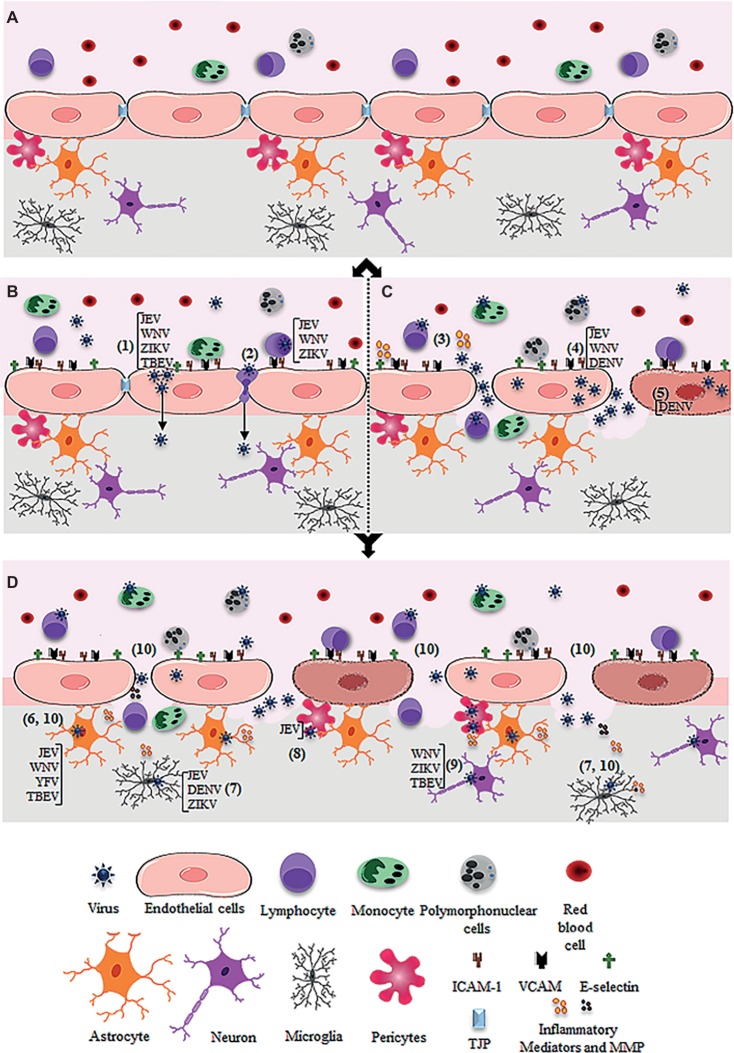
Figure 1.
Schematic figure showing possible mechanism of virus entry into the central nervous system through the blood-brain barrier. (A) Intact blood-brain barrier is composed by endothelial cell strongly adhered through tight junction proteins (TJP), in association to pericytes, astrocytes, and microglia. The barrier controls the flux of solutes, blood cells (lymphocytes, monocytes, and polymorphonuclear cells), and pathogens from the blood to the central nervous system. (B) After systemic infection, some flavivirus reaches the BBB through hematogenous route and may cross the endothelial barrier without remarkable cytopathic effect. JEV, WNV, ZIKV, and TBEV may cross the endothelial barrier as cell-free virus (1). JEV, WNV, and ZIKV were also reported to traverse the endothelial barrier associated to infected leukocytes (2). (C) Systemic infection and inflammation and/or the direct infection of brain endothelial cells may induce BBB breakdown, allowing virus invasion of the CNS. Regarding this, systemic inflammation due to activation of immune cells upon infection was associated to the release of inflammatory mediators, which then affect the permeability of the endothelial barrier (3). Also, replication of JEV, WNV, and DENV in the brain endothelial cells may induce downregulation of TJP expression (4) and/or cell death (5), promoting the barrier disruption and virus entry. (D) After entry into the brain by any of the described pathways (B and C), flaviviruses may infect the astrocytes (as described for JEV, WNV, YFV, TBEV) (6), microglia (as described for JEV, DENV, ZIKV) (7), pericytes (as described for JEV) (8), and neurons (as described for WNV, ZIKV, TBEV) (9). The infection of these cells, especially astrocytes and microglia, induces the release of inflammatory mediators (IL-6, VEGF, TNF-α, IFN-γ, IL-1β and IL-10, MCP-1) and metalloproteinases (MMP2, MMP3, MM9) (10), which mediate the downregulation of adherents and tight junction proteins (TJP), resulting in increased permeability.