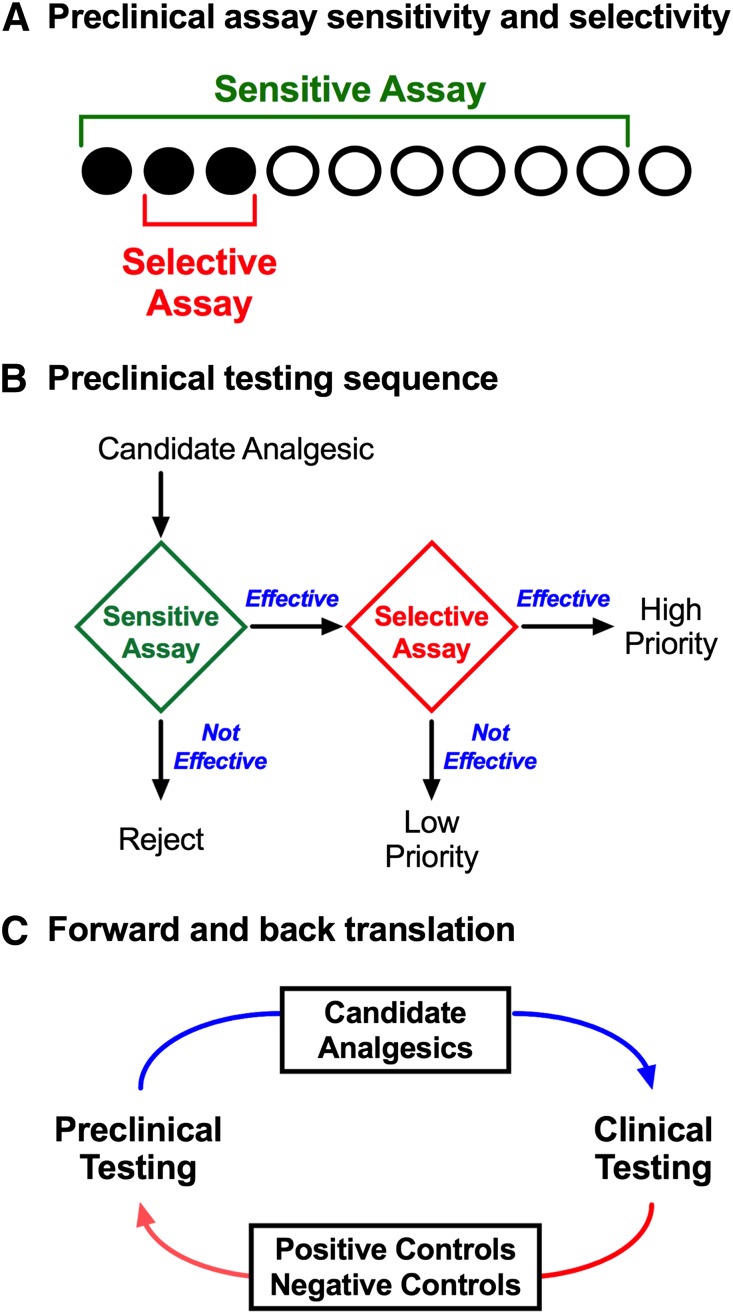
Fig. 1.
Translational research involves an iterative process of forward and back translation to use and refine procedures that optimize sensitivity and selectivity for analgesic drugs. (A) A theoretical array of drugs that will ultimately function in humans as effective analgesics (filled circles) or as ineffective analgesics (open circles). The challenge in preclinical research is to identify the effective analgesics and filter out the ineffective compounds. Sensitive assays (green bracket) detect all or most true-positive analgesics but are vulnerable to false-positive effects with drugs that are not analgesics. Selective assays (red bracket) detect some or most true-positive analgesics and are less vulnerable to false positives with nonanalgesics; however, selective assays may yield false-negative results that fail to detect some analgesics. (B) A strategy to sequence assays with complementary characteristics of sensitivity and selectivity and prioritize candidate analgesics for human testing. An initial screen with a sensitive assay can identify a subset of drugs that includes both many true-positive analgesics but also some false positives. Additionally, these assays can be used to characterize attributes of in vivo pharmacology, including potency, efficacy, time course, and receptor mechanism(s) of action. Secondary testing with a selective assay can then screen out many false positives to identify high-priority drugs for advancement to human testing. Drugs effective in the sensitive assay but not in the selective assay may still have potential as analgesics (i.e., they may be false negatives in the selective assay), but such drugs would have lower priority for advancement. Drugs that are not effective in the sensitive assay can be rejected. (C) The iterative process of forward and back translation for use and refinement of preclinical procedures. In forward translation, candidate analgesics are prioritized for advancement to clinical studies. In back translation, drugs identified as effective or ineffective analgesics can be tested as positive or negative controls, respectively, and can also be used to refine preclinical procedures in ways that increase sensitivity to the positive controls and selectivity against negative controls.