Figure 5.
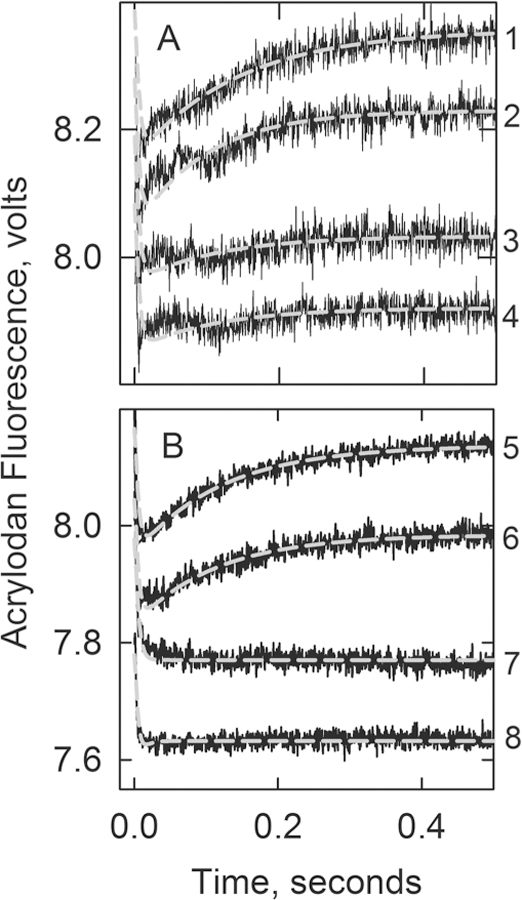
Acrylodan-labeled tropomyosin fluorescence changes following the rapid detachment of myosin S1 in the absence of Ca2+ at 10 °C: curve 1, actin filaments with wild-type troponin; curve 2, A8V TnC; curve 3, A8V TnC and Δ14 TnT; curve 4, Δ14 TnT. The gray lines are best fits of differential equations describing Scheme 1 to the data (see Experimental Procedures). Panel A included 4 μM actin, 0.86 μM tropomyosin, 0.86 μM troponin, and 2.5 μM S1 in 20 mM MOPS, 152 mM KCl, 4 mM MgCl2, 1 mM dithiothreitol, 2 mM EGTA rapidly mixed with 4 mM ATP, 20 mM MOPS, 132 mM KCl, 8 mM MgCl2, 1 mM dithiothreitol and 2 mM EGTA. Values of k7 + k8: 7.8 s−1 for the wild type and 7.7 s−1 for A8V. Amplitudes: 0.17 ± 0.05 V for the wild type, 0.15 ± 0.03 V for A8V, and <0.02 for Δ14 TnT and the double mutant. Panel B included 2 μM actin, 0.86 μM troponin, 0.86 μM tropomyosin, 2 μM S1 in 90 mM KCl, 20 mM MOPS buffer (pH 7), 4 mM MgCl2, 2 mM EGTA, and 1 mM dithiothreitol rapidly mixed with 2 mM ATP in 90 mM KCl, 20 mM MOPS buffer (pH 7), 4 mM MgCl2, 2 mM EGTA, and 1 mM dithiothreitol at 10 °C. Values of k7 + k8: 8.1 s−1 for the wild type and 7.7 s−1 for A8V. Amplitudes: 0.17 ± 0.02 V for the wild type and 0.13 ± 0.01 V for A8V.
