Figure 1. Model of the Brucella intracellular cycle in macrophages.
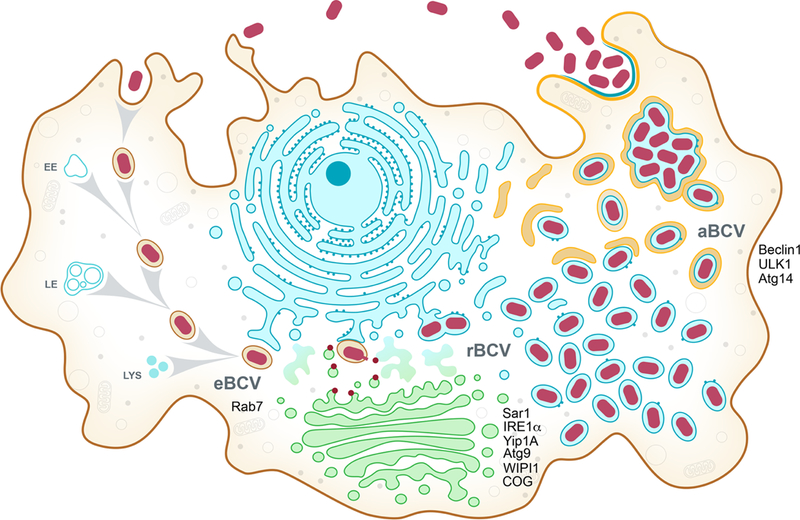
Following phagocytic uptake by macrophages, Brucella spp. reside in the first 8 to 12 h post infection within a membrane bound vacuole that undergoes endosomal maturation via sequential interactions with early (EE) and late (LE) endosomes and lysosomes (LYS) to become an acidified, endosomal Brucella-containing vacuole (eBCV). The host small GTPase Rab7 contributes to eBCV maturation, which provides physicochemical cues promoting expression of the VirB Type IV secretion system (T4SS), which translocates effector proteins (red) that mediate eBCV interactions with ER exit site and acquisition of ER and Golgi-derived membranes. These events lead to the biogenesis of replication permissive, ER-derived BCVs, called replicative BCVs (rBCVs). The host proteins Sar1, IRE1α, Yip1A, Atg9, WIPI1 and the COG complex contribute to rBCV biogenesis. Bacteria then undergo extensive replication in rBCVs between 12 and 48 h pi, after which rBCVs are captured within autophagosome-like structures in a VirB T4SS-dependent manner, to become autophagic BCVs (aBCVs). aBCV formation requires the host autophagy proteins Beclin1, ULK1 and Atg14. aBCVs harbors features of autolysosomes and are required for bacterial egress and new cycles of intracellular infections.
