Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HH
hemochromatosis
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
Evidence of the Role of Iron Overload in Hepatocellular Carcinoma
A direct role of iron in hepatocarcinogenesis has been suggested on the basis of the evidence that patients with hereditary hemochromatosis (HH) had a 200‐fold greater risk of developing hepatocellular carcinoma (HCC) than the general population.1 The risk of HCC developing in HH patients is higher than that of patients with non–HH‐related chronic liver diseases, matched for sex, age, and severity of liver fibrosis.2 It has been shown in case‐control studies that African subjects with dietary iron overload have a higher HCC risk as well.3 Moreover, HCC has been described in several patients with transfusion‐associated iron overload or iron overload related to ineffective erythropoiesis.4
Mechanisms of Iron Toxicity in HCC
One of the major, as yet unsolved dilemmas is whether iron exerts its oncogenic potential indirectly, through the induction of cirrhosis, or whether it also has direct carcinogenic activity. The possibility that free iron can exert an oncogenic potential is related to its ability, being electronically unstable, to induce oxidative stress. Reactive oxygen species production, in physiologic conditions controlled by antioxidant intracellular defense mechanisms, can lead to lipid peroxidation and oxidative damage to several cellular membranes and organelles, including DNA. Thus, excess free iron with reactive oxygen species overproduction in hepatic tissue, exceeding the cellular defenses, could be responsible for mutagenesis and hepatocarcinogenesis, overcoming the protective effect of activation of tumor suppressor genes and critical DNA repair genes orchestrated by p535, 6, 7 (Fig. 1). This implies that, even in the presence of minor degrees of hepatic iron excess, patients may be exposed to an increased risk of cancer. Experimental evidence also supports a role for iron in HCC development in animal models, even in the absence of cirrhosis,8 thereby suggesting that iron has a direct effect on HCC. This is further supported by the observation that patients with noncirrhotic HH may develop HCC, albeit rarely.
Figure 1.
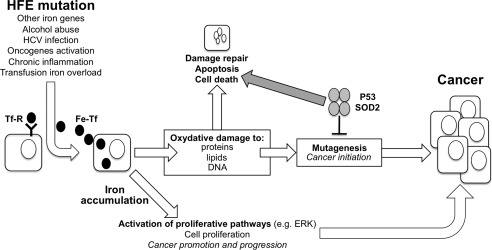
The hypothesized mechanisms of iron toxicity in hepatic carcinogenesis. The free iron induces oxidative stress with overproduction of reactive oxygen species, that, exceeding the physiological cellular defenses, can lead to lipid peroxidation and oxidative damage to several cellular membranes and organelles, including DNA.
Iron as a Cofactor in the Pathogenesis of HCC
It has also been suggested that iron plays a role in HCC development in chronic liver diseases associated with minor iron overload/siderosis. Among conditions associated with minor degrees of increased iron deposition, the more prevalent include chronic hepatitis C virus (HCV) hepatitis, alcoholic liver disease and nonalcoholic fatty liver disease (NAFLD) associated with insulin resistance and the metabolic syndrome (Table 1). The most widely held hypothesis is that oxidative stress can be induced by these different etiologies and that liver iron promotes injury and fibrosis acting synergistically with the initial etiologic insult.9, 10 Interestingly, it has been shown that HCV is able to interfere with iron metabolism, inhibiting hepatic transcription of hepcidin, the key iron metabolism regulatory hormone, which is in turn followed by increased liver iron deposition.11 Less well‐defined is the interaction of alcohol and NAFLD with hepcidin, with conflicting evidence supporting or refuting an inhibiting effect on hepcidin transcription (Fig. 2).
Figure 2.
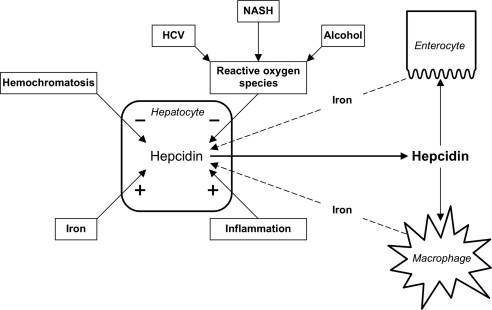
The role of iron in liver disease. Oxidative stress in the liver can be induced by HCV, alcohol, NASH, hemochromatosis, and inflammation. Oxidative stress can depress the expression of hepcidin, the key regulator of iron metabolism that controls duodenal iron absorption and iron release from macrophages.
To address the possible wider role of iron in hepatocarcinogenesis, several authors have studied HFE, the gene responsible for most cases of HH, in diverse liver diseases. In patients with chronic liver disease and mild iron overload, conflicting results concerning a possible association between HFE status and HCC have been reported (Table 2).12, 13, 14, 15 In a prospective study of 301 consecutive patients with cirrhosis, it was found that liver iron overload and heterozygosity for the C282Y HFE mutation were associated with a higher risk of HCC in patients with alcoholic but not HCV‐related cirrhosis.16 In addition, it has been shown that patients with polymorphisms of oxidant/antioxidant enzymes were at increased risk of cancer.17 Similarly, it has been reported that the risk of HCC is higher in patients who have nonalcoholic steatohepatitis (NASH)–related cirrhosis with hepatic iron excess.18 However, the absence of a clear association in NAFLD between iron accumulation and HFE mutations means that there is no value in assessing risk of HCC in NASH‐related cirrhosis by testing for HFE mutations.19
Iron Depletion as a Therapeutic Option
It is an open question whether iron depletion therapy, either by phlebotomy or iron chelation, may reduce the risk of HCC. In the only available prospective study, which included a small series of patients with HCV‐related cirrhosis, patients who underwent phlebotomy had a reduced risk of HCC.20 This study needs to be confirmed in a prospective longitudinal well‐powered study. Interestingly, iron depletion by phlebotomy was associated with a reduced risk of developing cancer in a randomized trial including a large series of subjects with peripheral vascular disease.21
In conclusion, iron excess may be considered a potential oncogenic factor. Controlled clinical studies are needed to propose iron depletion as a HCC preventive therapy.
Potential conflict of interest: Nothing to report.
References
- 1. Niederau C, Fischer R, Pürschel A, Stremmel W, Häussinger D, Strohmeyer G. Long‐term survival in patients with hereditary hemochromatosis. Gastroenterology 1996;110:1107‐1119. [DOI] [PubMed] [Google Scholar]
- 2. Fracanzani AL, Conte D, Fraquelli M, Taioli E, Mattioli M, Losco A, et al. Increased cancer risk in a cohort of 230 patients with hereditary hemochromatosis in comparison to matched control patients with non‐iron‐related chronic liver disease. Hepatology 2001;33:647‐651. [DOI] [PubMed] [Google Scholar]
- 3. Kew MC. Hepatocellular carcinoma in African blacks: recent progress in etiology and pathogenesis. World J Hepatol 2010;2:65‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mancuso A. Hepatocellular carcinoma in thalassemia: a critical review. World J Hepatol 2010;2:171‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asare GA, Mossanda KS, Kew MC, Paterson AC, Kahler‐Venter CP, Siziba K. Hepatocellular carcinoma caused by iron overload: a possible mechanism of direct hepatocarcinogenicity. Toxicology 2006;219:41‐52. [DOI] [PubMed] [Google Scholar]
- 6. Fargion S, Valenti L, Fracanzani AL. Beyond hereditary hemochromatosis: new insights into the relationship between iron overload and chronic liver diseases. Dig Liver Dis 2011;43:89‐95. [DOI] [PubMed] [Google Scholar]
- 7. Dongiovanni P, Fracanzani AL, Cairo G, Megazzini CP, Gatti S, Rametta R, et al. Iron‐dependent regulation of MDM2 influences p53 activity and hepatic carcinogenesis. Am J Pathol 2010;176:1006‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kew MC. Hepatic iron overload and hepatocellular carcinoma. Cancer Lett 2009;286:38‐43. [DOI] [PubMed] [Google Scholar]
- 9. Fujinaga H, Tsutsumi T, Yotsuyanagi H, Moriya K, Koike K. Hepatocarcinogenesis in hepatitis C: HCV shrewdly exacerbates oxidative stress by modulating both production and scavenging of reactive oxygen species. Oncology 2011;81(suppl 1):11‐17. [DOI] [PubMed] [Google Scholar]
- 10. Asare GA, Bronz M, Naidoo V, Kew MC. Synergistic interaction between excess hepatic iron and alcohol ingestion in hepatic mutagenesis. Toxicology 2008;254:11‐18. [DOI] [PubMed] [Google Scholar]
- 11. Trinder D, Ayonrinde OT, Olynyk JK. HCV, iron, and oxidative stress: the new choreography of hepcidin. Gastroenterology 2008;134:348‐351. [DOI] [PubMed] [Google Scholar]
- 12. Pirisi M, Toniutto P, Uzzau A, Fabris C, Avellini C, Scott C, et al. Carriage of HFE mutations and outcome of surgical resection for hepatocellular carcinoma in cirrhotic patients. Cancer 2000;89:297‐302. [DOI] [PubMed] [Google Scholar]
- 13. Fracanzani AL, Fargion S, Stazi MA, Valenti L, Amoroso P, Cariani E, et al. Association between heterozygosity for HFE gene mutations and hepatitis viruses in hepatocellular carcinoma. Blood Cells Mol Dis 2005;35:27‐32. [DOI] [PubMed] [Google Scholar]
- 14. Jin F, Qu LS, Shen XZ. Association between C282Y and H63D mutations of the HFE gene with hepatocellular carcinoma in European populations: a meta‐analysis. J Exp Clin Cancer Res 2010;29:18‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Motawi TK, Shaker OG, Ismail MF, Sayed NH. Genetic variants associated with the progression of hepatocellular carcinoma in hepatitis C Egyptian patients. Gene 2013;527:516‐520. [DOI] [PubMed] [Google Scholar]
- 16. Nahon P, Sutton A, Rufat P, Ziol M, Thabut G, Schischmanoff PO, et al. Liver iron, HFE gene mutations, and hepatocellular carcinoma occurrence in patients with cirrhosis. Gastroenterology 2008;134:102‐110. [DOI] [PubMed] [Google Scholar]
- 17. Nahon P, Sutton A, Rufat P, Ziol M, Akouche H, Laguillier C, et al Myeloperoxidase and superoxide dismutase 2 polymorphisms comodulate the risk of hepatocellular carcinoma and death in alcoholic cirrhosis. Hepatology 2009;50:1484‐1493. [DOI] [PubMed] [Google Scholar]
- 18. Sorrentino P, D'Angelo S, Ferbo U, Micheli P, Bracigliano A, Vecchione R. Liver iron excess in patients with hepatocellular carcinoma developed on non‐alcoholic steato‐hepatitis. J Hepatol 2009;50:351‐357. [DOI] [PubMed] [Google Scholar]
- 19. Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E, et al. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2010;138:905‐912. [DOI] [PubMed] [Google Scholar]
- 20. Kato J, Miyanishi K, Kobune M, Nakamura T, Takada K, Takimoto R, et al. Long‐term phlebotomy with low‐iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol 2007;42:830‐836. [DOI] [PubMed] [Google Scholar]
- 21. Zacharski LR, Shamayeva G, Chow BK. Effect of controlled reduction of body iron stores on clinical outcomes in peripheral arterial disease. Am Heart J 2011;162:949‐957. [DOI] [PubMed] [Google Scholar]


