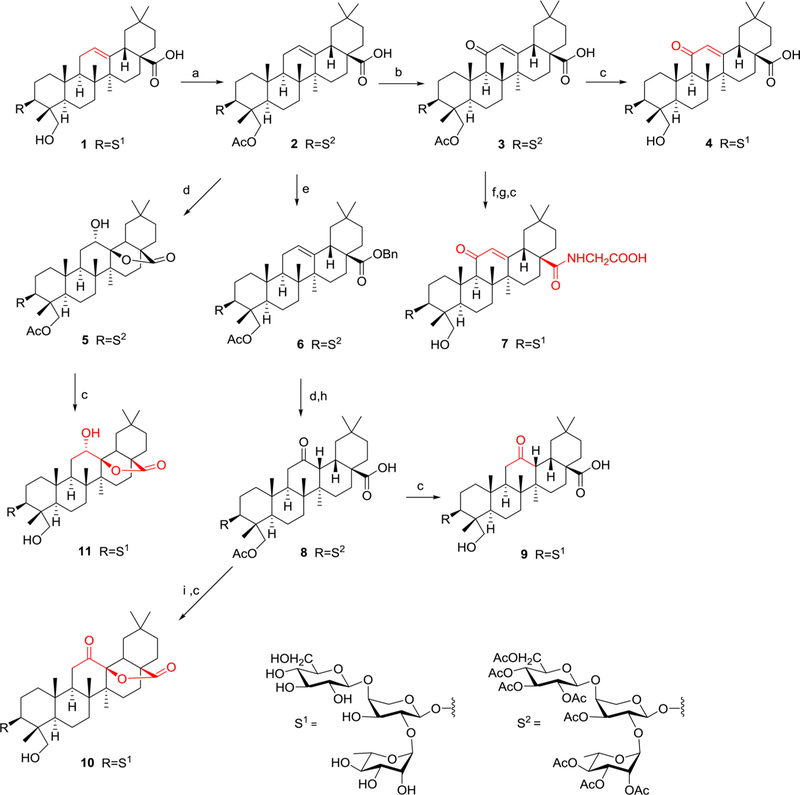
Scheme 1.
Synthesis of 2−11 from 1a, aReagents and conditions: (a) Ac2O, py, rt, 4 h, 95%; (b) K2CrO4, glacial acetic acid, reflux, 6 h, 80%; (c) KOH, MeOH/THF/H2O, rt, overnight; (d) m-CPBA, CHCl3, dark, 2 days; (e) BrBn, K2CO3, DMF, rt, 8 h, 95%; (f) (COCl)2, CH2Cl2, rt, 6 h; (g) NH2CH2COOCH2CH3 HCl, CH2Cl2, Et3N, rt, overnight; (h) H2, 10% Pd/C, THF, 6 h; (i) DDQ, anhydrous benzene, 90 °C, 2 days.