Table 1.
Optimization of the stereodivergent asymmetric Mannich reaction.
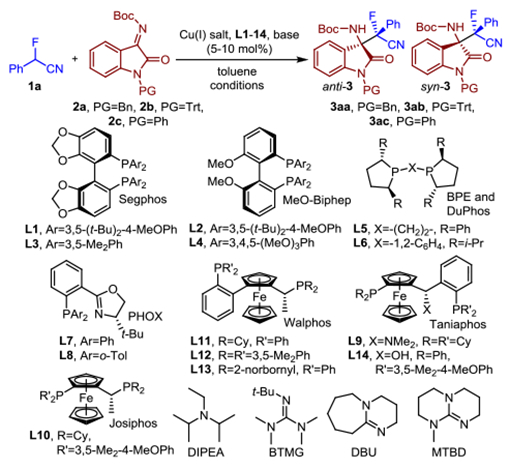 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Cu(I) source | Ligand | 2 | Conditions | Yield (%) | dra (anti/syn) | eeb (%) |
| 1 | Cu(PhMe)0.5OTf | L1 | 2a | DIPEAc, 25 °C | 91 | 1.7:1 | 41 |
| 2 | Cu(PhMe)0.5OTf | L1 | 2b | DIPEAc, 25 °C | 85 | 5.2:1 | 80 |
| 3 | Cu(PhMe)0.5OTf | L2 | 2b | DIPEAc, 25 °C | 74 | 4.6:1 | 75 |
| 4 | Cu(PhMe)0.5OTf | L1 | 2b | BTMG, 25 °C | 98 | 5.8:1 | 83 |
| 5 | Cu(MeCN)4PF6 | L1 | 2b | BTMG, 25 °C | 98 | 6.7:1 | 83 |
| 6 | Cu(MeCN)4PF6 | L1 | 2b | DBU, 25 °C | 99 | 6.6:1 | 73 |
| 7 | Cu(MeCN)4PF6 | L1 | 2b | MTBD, 25 °C | 99 | 5.8:1 | 83 |
| 8 | Cu(MeCN)4PF6 | L3 | 2b | BTMG, 25 °C | 99 | 2.0:1 | 26 |
| 9 | Cu(MeCN)4PF6 | L4 | 2b | BTMG, 25 °C | 99 | 4.0:1 | 79 |
| 10 | Cu(MeCN)4PF6 | L5 | 2b | BTMG, 25 °C | 99 | 1.5:1 | 50 |
| 11 | Cu(MeCN)4PF6 | L6 | 2b | BTMG, 25 °C | 99 | 1.5:1 | 31 |
| 12 | Cu(MeCN)4PF6 | L7 | 2b | BTMG, 25 °C | 99 | 1.3:1 | 40 |
| 13 | Cu(MeCN)4PF6 | L8 | 2b | BTMG, 25 °C | 99 | 1.1:1 | 47 |
| 14 | Cu(MeCN)4PF6 | L1 | 2b | BTMG, −35 °C | 95 | 12.3:1 | 90 |
| 15d | Cu(MeCN)4PF6 | L9 | 2b | BTMG, 25 °C | 97 | 1:1.6 | 3 |
| 16d | Cu(MeCN)4PF6 | L9 | 2a | BTMG, 25 °C | 99 | 1:5.7 | 31 |
| 17d | Cu(MeCN)4PF6 | L10 | 2a | BTMG, −35 °C | 75 | 1:2.5 | 4 |
| 18d | Cu(MeCN)4PF6 | L11 | 2a | BTMG, −35 °C | 99 | 1:7.3 | 19 |
| 19d | Cu(MeCN)4PF6 | L12 | 2a | BTMG, −35 °C | 99 | 1:5.6 | 60 |
| 20d | Cu(MeCN)4PF6 | L13 | 2a | BTMG, −35 °C | 99 | 1:13.4 | 70 |
| 21d | Cu(MeCN)4PF6 | L13 | 2c | BTMG, −35 °C | 98 | 1:8.7 | 75 |
| 22d | Cu(MeCN)4PF6 | L14 | 2a | BTMG, −35 °C | 94 | 1:5.7 | 80 |
| 23d | Cu(MeCN)4PF6 | L14 | 2c | BTMG, −35 °C | 94 | 1:3.0 | 98 |
Reaction condition: 1a (0.055 mmol), 2 (0.050 mmol), Cu(I) source (0.005 mmol), ligand (0.006 mmol) and base in 0.3 mL toluene.
Determined by 19F NMR analysis.
Determined by chiral HPLC analysis.
The base loading was 80 mol%.
The Cu complex and base loading were 5 mol%.
