Figure 3.
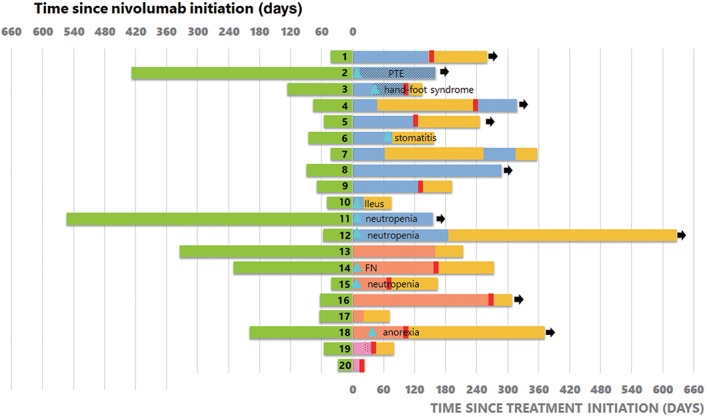
Treatment duration of nivolumab and ramucirumab (RAM) plus docetaxel (DTX) in all patients. Nine out of 20 patients experienced grade 3 adverse events. The treatment duration of nivolumab was < 90 days in 9 (75%) patients with a partial response (PR), 3 (50%) patients with stable disease (SD) and 2 (100%) patients with progressive disease (PD) on RAM + DTX. FN, febrile neutropenia; OS, overall survival; PTE, pulmonary thromboembolism.  , Time receiving Nivolumab;
, Time receiving Nivolumab;  , Time receiving DTX+RAM (PR Group);
, Time receiving DTX+RAM (PR Group);  , Time receiving DTX+RAM (SD Group);
, Time receiving DTX+RAM (SD Group);  , Time receiving DTX+RAM (PD Group) (dots DTX only);
, Time receiving DTX+RAM (PD Group) (dots DTX only);  , OS not receiving DTX+RAM;
, OS not receiving DTX+RAM;  , Progressive disease;
, Progressive disease;  , Adverse event, ≧Grade3;
, Adverse event, ≧Grade3;  , Alive as of database lock.
, Alive as of database lock.
