Figure 3.
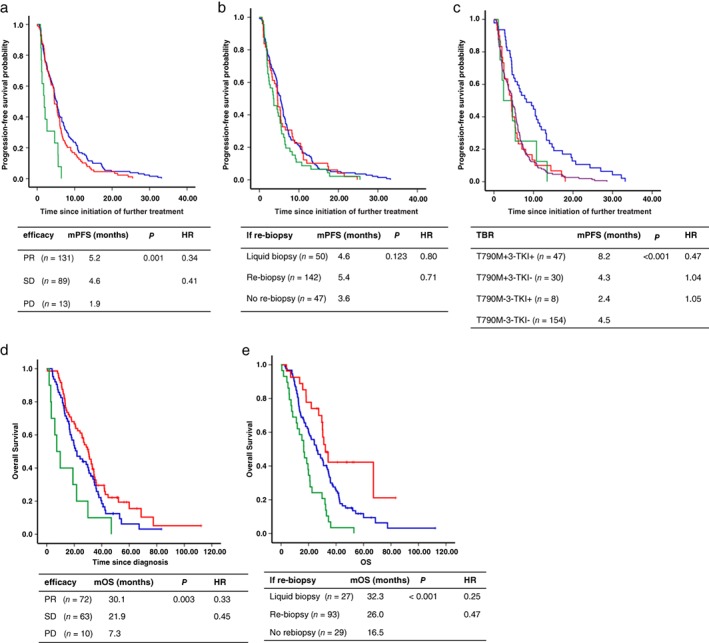
Kaplan–Meier estimates of the duration of progression‐free survival (PFS) of further treatment in (a) patients who exhibited an objective response to EGFR‐tyrosine kinase inhibitors (TKIs), efficacy ( ) PR, (
) PR, ( ) SD and (
) SD and ( ) PD, (b) patients who underwent re‐biopsy or not, if re‐biopsy (
) PD, (b) patients who underwent re‐biopsy or not, if re‐biopsy ( ) re‐biopsy, (
) re‐biopsy, ( ) liquid biopsy and (
) liquid biopsy and ( ) no biopsy and (c) patients divided into different groups according to whether T790M was positive and third‐generation TKIs (3‐TKIs) were administered, TBR (
) no biopsy and (c) patients divided into different groups according to whether T790M was positive and third‐generation TKIs (3‐TKIs) were administered, TBR ( ) T790M +3‐TKI+, (
) T790M +3‐TKI+, ( ) T790M +3‐TKI−, (
) T790M +3‐TKI−, ( ) T790M −3‐TKI+ and (
) T790M −3‐TKI+ and ( ) T790M −3‐TKI−. Kaplan–Meier estimates of the duration of overall survival (OS) of (d) patients who exhibited an objective response to EGFR‐TKIs, efficacy (
) T790M −3‐TKI−. Kaplan–Meier estimates of the duration of overall survival (OS) of (d) patients who exhibited an objective response to EGFR‐TKIs, efficacy ( ) PR, (
) PR, ( ) SD, (
) SD, ( ) PD, (
) PD, ( ) PR‐censored, (
) PR‐censored, ( ) SD‐censored and (
) SD‐censored and ( ) PD‐censored and (e) patients who underwent re‐biopsy or not, if re‐biopsy (
) PD‐censored and (e) patients who underwent re‐biopsy or not, if re‐biopsy ( ) re‐biopsy, (
) re‐biopsy, ( ) liquid biopsy, (
) liquid biopsy, ( ) no rebiopsy, (
) no rebiopsy, ( ) re‐biopsy‐censored, (
) re‐biopsy‐censored, ( ) liquid biopsy‐censored and (
) liquid biopsy‐censored and ( ) no rebiopsy‐censored. HR, hazard ratio; mOS, median OS; mPFS, median PFS; mut plus T790M, patients harbored a baseline mutation and a T790M mutation when re‐tested; PD, progressive disease; PR, partial response; SD, stable disease; T790M, patients only harbored a T790M mutation and the baseline sensitive mutation disappeared.
) no rebiopsy‐censored. HR, hazard ratio; mOS, median OS; mPFS, median PFS; mut plus T790M, patients harbored a baseline mutation and a T790M mutation when re‐tested; PD, progressive disease; PR, partial response; SD, stable disease; T790M, patients only harbored a T790M mutation and the baseline sensitive mutation disappeared.
