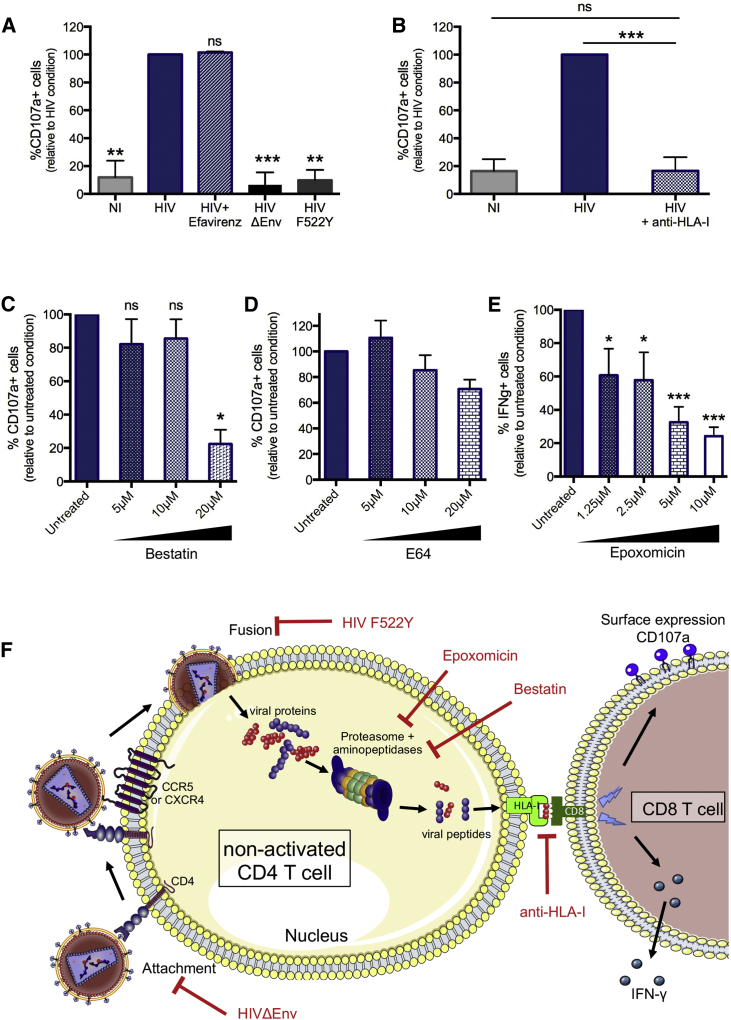
Figure 4.
CD8+ T Cell Response Induced by the Presentation of Viral Peptides from Incoming HIV Particles in Non-activated CD4+ T Cells through the HLA-I Molecule
(A) The CD8+ T cell response to HIV+ non-activated CD4+ T cells was evaluated by flow cytometry with CD107a staining without HIV Env-CD4 and co-receptor interactions (HIV ΔEnv), without HIV entry into CD4+ T cells (HIV F522Y), or in the presence of a reverse transcription inhibitor (efavirenz) and compared to HIV infection alone (HIV).
(B) The importance of the HLA-I molecule in the CD8+ T cell response was evaluated by blocking the HLA-I molecule with the anti-class I antibody (clone W6/32), and measuring degranulation.
(C–E) The role of the antigen-processing pathway in CD8+ T cell recognition was evaluated by measuring degranulation by flow cytometry. This was done by blocking the aminopeptidases with bestatin (C), the cysteine proteases with E64 (D), and the proteasome with epoxomicin (E).
(F) Schematic representation of the required steps from viral entry to antigen presentation in a non-activated CD4+ T cell to induce a CD8+ T cell response. Means ± SDs for three independent experiments with cells coming from three different HIV controllers are represented.
Statistics were calculated with ANOVA multiple comparison tests relative to the HIV condition for (A), each column relative to every other column for (B), and relative to the untreated condition for (C)–(E).
∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. Regarding epoxomicin, the drug induced CD107a surface expression on CD8+ T cells by itself through an unknown mechanism, but in the absence of HIV peptide stimulation or HIV exposure (data not shown). For this reason, IFN-γ staining was used.