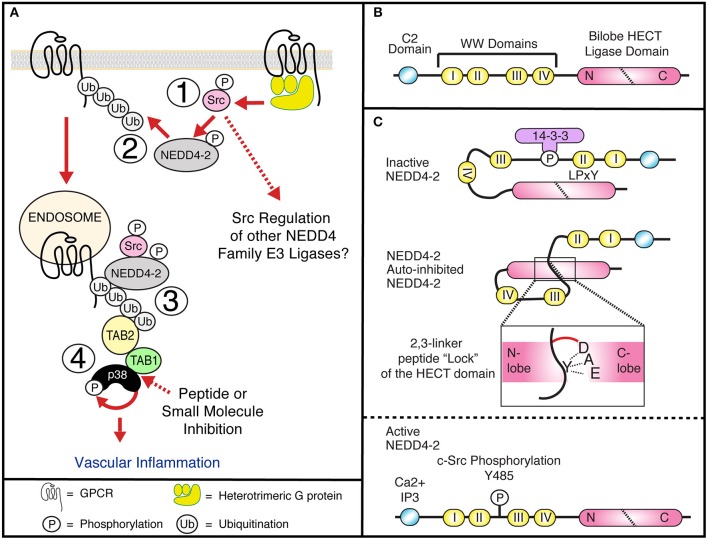
Figure 1.
(A1) GPCRs induce Gαq dependent c-Src activation at the plasma membrane and tyrosine phosphorylation of NEDD4-2, releasing NEDD4-2 auto-inhibition. (A2) Activated NEDD4-2 ubiquitinated GPCRs. (A3) Internalized, endosomal GPCRs recruit the adaptor proteins TAB1 and TAB2, to active p38 through an atypical pathway. (A4) GPCR induced atypical p38 signaling regulates proinflammatory signaling in vasculature. The interface between TAB1 and p38 is a potential druggable target for therapeutic intervention. (B) NEDD4-2 contains a C2 lipid and Calcium binding domain, four WW domains that bind PP/LPxY motifs and a C-terminal bilobed Catalytic HECT domain. (C) NEDD4-2 can be held in an inactive state by serine phosphorylation dependent recruitment of 14-3-3 (Debonneville et al., 2001; Bhalla et al., 2005; Ichimura et al., 2005), and intramolecular binding of WWII with a LPxY motif on the HECT domain (Bruce et al., 2008; Escobedo et al., 2014), or through binding of an auto-inhibitory 2,3-linker peptide that wraps the N-lobe of the HECT domain locking the ligase in a closed confirmation. Active NEDD4-2 requires dephosphorylation and release of 14-3-3, calcium and lipid binding to the C2 domain and tyrosine phosphorylation of Y485 (Grimsey et al., 2018).