Figure 2. The focal adhesion clutch.
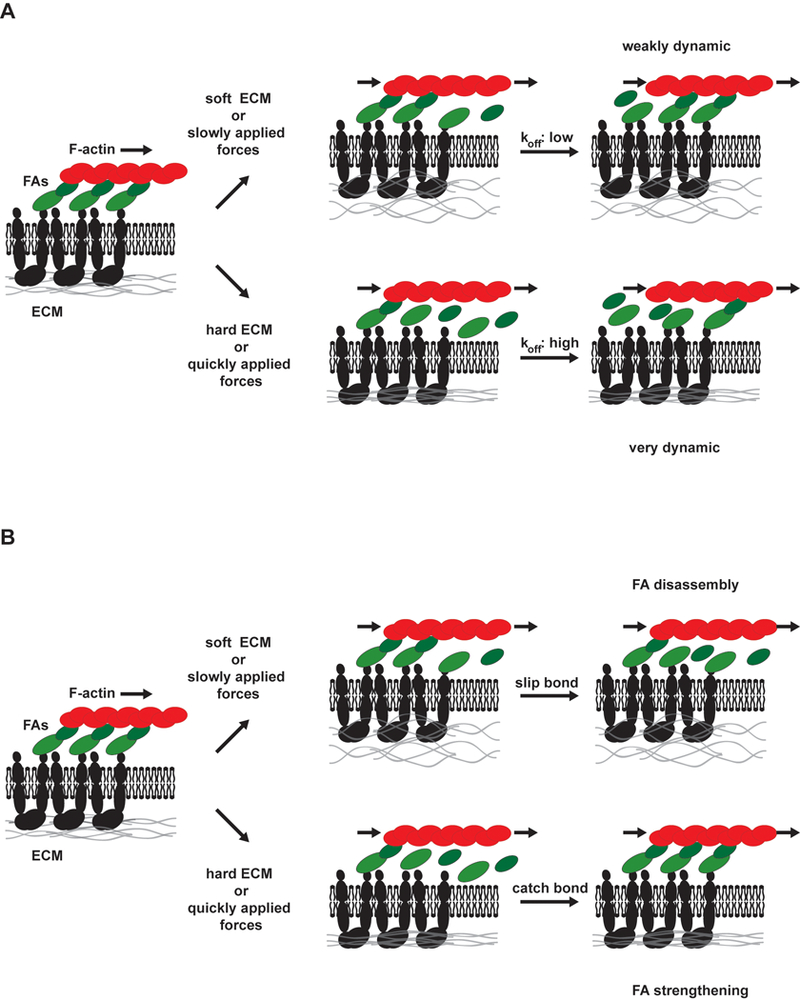
Due to forces from actin polymerization and myosin-dependent contractility, actin filaments flow rearward over FAs toward the nucleus. Through FA proteins that link actin to integrins, force is applied to the ECM. Force-dependent changes in FA exchange rates (represented as green arrows, see Box 1 for details) alter dynamics and size of FAs. On soft surfaces or where external force is applied slowly, on- and off-rates are moderately fast and FAs have moderate lifetimes. However, on stiff surfaces or when external forces are applied quickly, distinct behaviors can be observed. In the absence of FA strengthening, molecular linker dissociation rates increase (slip bond behavior). However, these proteins are sometimes replaced through re-binding, resulting in large exchange rates and short-lived FAs. FA strengthening is associated with catch bonds that slow FA protein dissociation under force, with exposure of cryptic binding sites to recruit new proteins that reinforce the adhesion, and with conformational changes in FA proteins that activate signaling pathways to recruit additional molecular linkers. Any way, large, long-lived FAs are the result.
