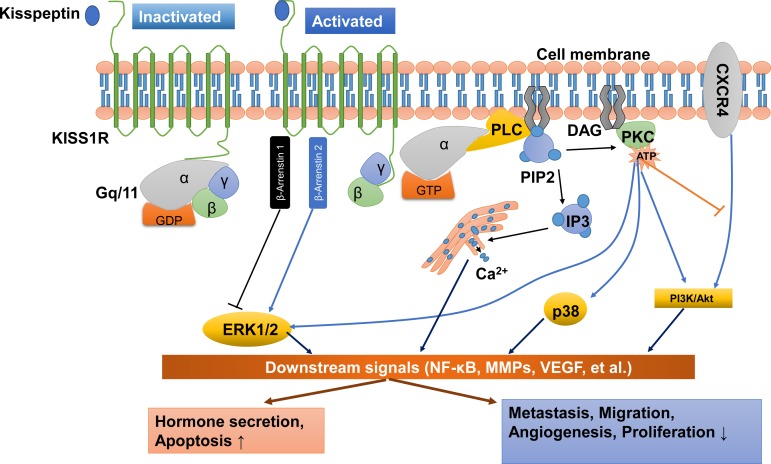
Figure 2.
Kisspeptin/KISS1R signaling and potential functions at a glance. KISS1R is a seven-transmembrane domain, Gq/11-coupled receptor. Upon binding of kisspeptin, the intracellular portion of KISS1R phosphorylates Gq/11. The α-subunit of Gq/11 activates PLC, which subsequently cleaves PIP2 into IP3 and DAG. IP3 promotes intracellular Ca2+ release from the endoplasmic reticulum, while DAG activates a signaling cascade by phosphorylating PKC. PKC activation induces the phosphorylation of ERK1/2 and p38. In addition, activation of KISS1R recruits arrestin-1 and -2, which down-regulates and up-regulates phosphorylated ERK1/2 levels, respectively. The activation of KISS1R can also stimulate the phosphorylation of PI3K/Akt, and suppress the phosphorylation of PI3K/Akt by blocking the prometastatic chemokine receptor CXCR4 (the receptor for the chemokine stromal cell-derived factor 1) signaling. These signals act on downstream pathways, including NF-κB, MMPs and VEGF, to promote hormone secretion and apoptosis and inhibit metastasis, migration, angiogenesis and proliferation. DAG, diacylglycerol; ERK1/2, extracellular signal-regulated kinase; IP3, inositol 1,4,5-triphosphate; PI3K, phosphatidylinositol-3-kinase; MMPs, matrix metalloproteinases; NF-κB, nuclear factor κB; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PLC, phospholipase C; VEGF, vascular endothelial growth factor.