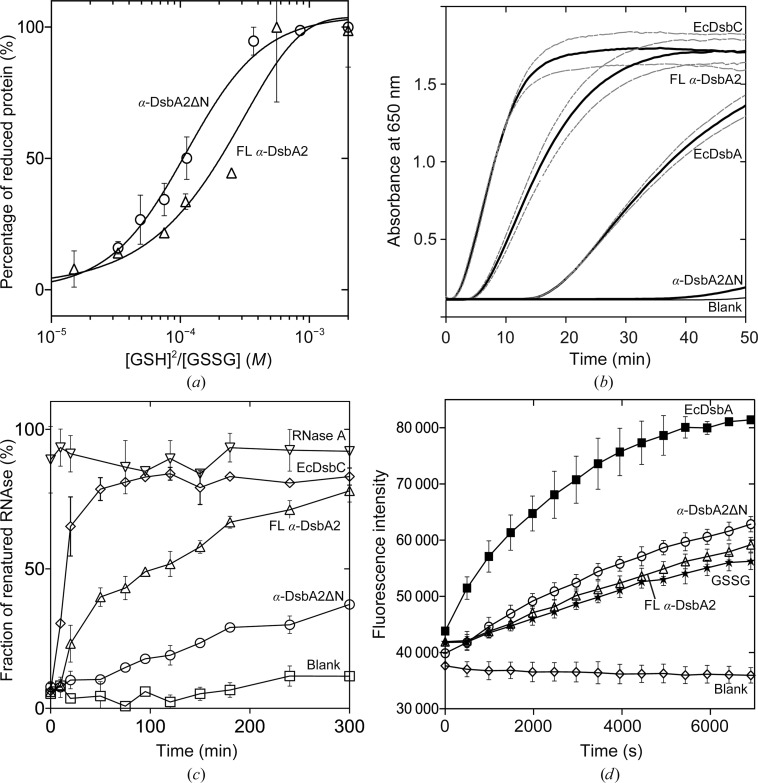
Figure 2.
Redox properties of α-DsbA2. (a) Redox potential. The equilibria between reduced and oxidized FL α-DsbA2 (triangles) and α-DsbA2ΔN (circles) are shown. Data are presented as the mean ± SD of two measurements. (b) Protein disulfide reductase activity. An insulin-reduction assay was performed to measure the ability of α-DsbA2 variants to reduce insulin. The reduction of insulin, catalyzed by the protein or noncatalyzed (blank), was monitored at 650 nm over 50 min. The mean (black line) and standard deviation error (light grey lines) of three replicate measurements are shown. Dithiol oxidase (EcDsbA) and disulfide isomerase (EcDsbC) activities are shown for comparison. (c) Protein disulfide isomerase activity. Isomerization of scrambled RNase A in the presence of EcDsbC (diamonds), FL α-DsbA2 (triangles) or α-DsbA2ΔN (circles), positive control (RNase A; inverted triangles) and blank (scrambled RNase A without enzyme; squares). Data are presented as the mean ± SD of three replicate measurements. (d) Dithiol oxidase activity. Fluorescence curves showing dithiol oxidation (in the presence of GSSG) of a model peptide by EcDsbA (positive control; squares), α-DsbA2ΔN (circles), FL α-DsbA2 (triangles), GSSG (without enzyme, i.e. negative control; stars) and α-DsbA2 without peptide (blank; diamonds). Data are presented as the mean ± SD of three replicate measurements.