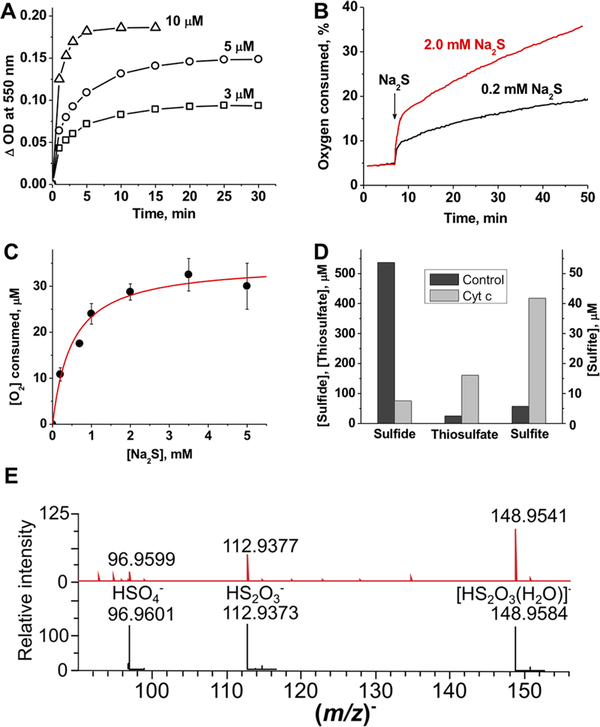
Figure 2.
Cyt C reduction kinetics and product analysis. (A) Kinetics of aerobic Cyt C reduction (10 μM) in the presence of 3, 5, and 10 μM Na2S2 in 100 mM HEPES buffer (pH 7.4) at 25 °C. Complete reduction with equimolar Na2S2 was seen within 5−10 min. (B) Kinetics of O2 consumption in the presence of Cyt C and sulfide. Addition of sulfide to Cyt C [50 μM in 100 mM HEPES buffer (pH 7.4)] at 25 °C results in rapid initial consumption of O2 followed by a slower phase. (C) Dependence of the concentration of O2 consumed in the burst phase on sulfide concentration. A hyperbolic fit to the data (mean ± SD of three to five independent experiments) gives a Kact of 0.5 ± 0.1 mM and a maximal O2 consumed of 35 ± 2 μM (red line), yielding a O2 consumed:Cyt C stoichiometry of 0.7:1.0. (D) Consumption of sulfide and accumulation of thiosulfate and sulfite 2 h after addition of 800 μM Na2S to 100 mM HEPES buffer (pH 7.4) without (black bars) or with (gray bars) 100 μM Cyt C at 25 °C under aerobic conditions. The initial levels of sulfide, thiosulfate, and sulfite were 756, 25, and 3.5 μM, respectively. (E) MS detection of sulfur products formed in the reaction of 10 μM Cyt C with 100 μM H2S in ammonium carbonate buffer (pH 7.7) under aerobic conditions. The reaction was monitored for 15 min at room temperature. The observed spectrum is at the top, and the simulated spectra with an isotopic distribution are below.