FIG 8.
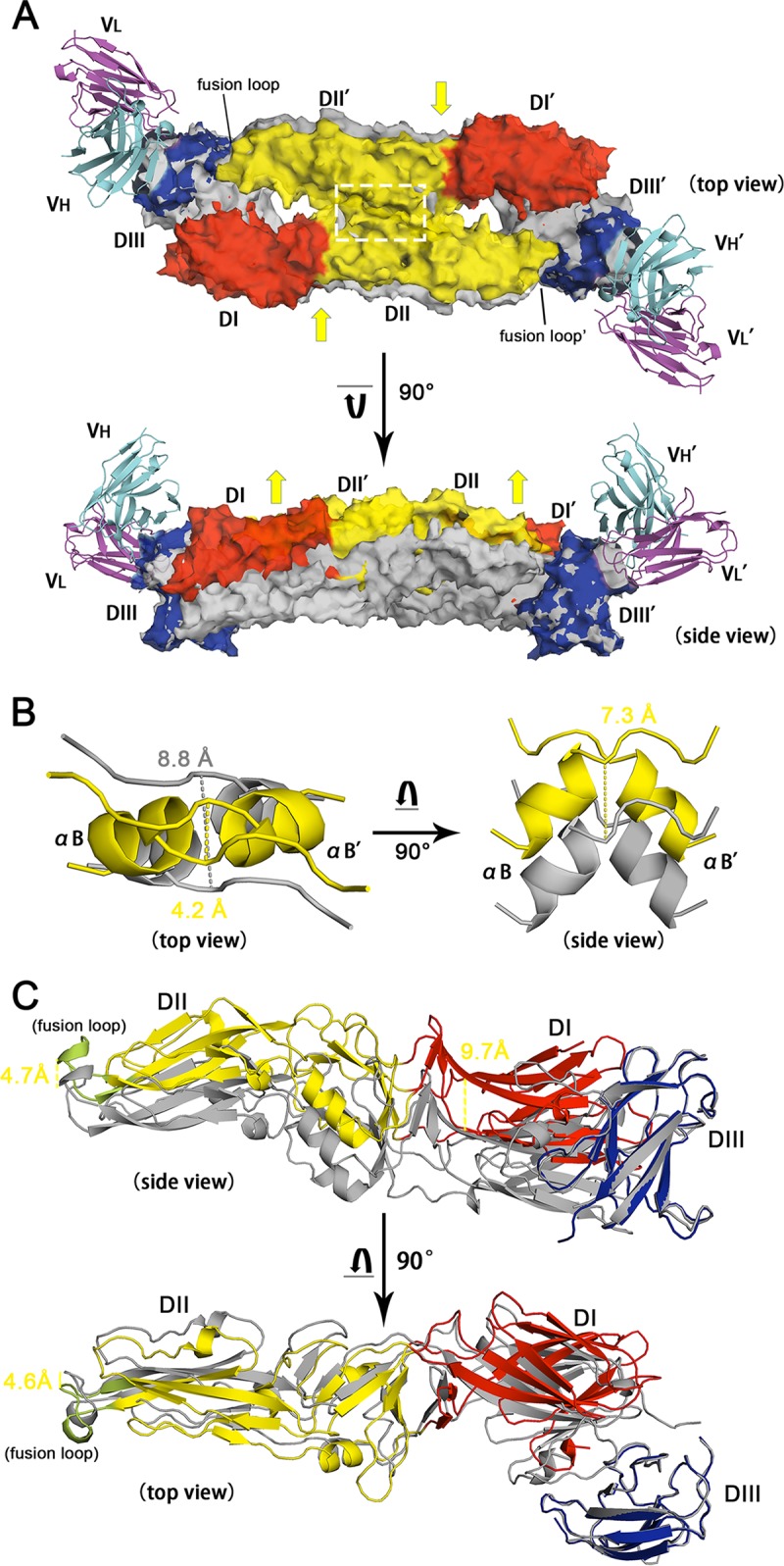
Structural comparison between the free and MAb 4.2 scFv-bound TBEV E homodimers. (A) Superimposition of TBEV E/MAb 4.2 scFv with the TBEV E dimer (PDB accession number 1SVB). The antibody complex is presented with various colors for each domain, as described in the legend to Fig. 4D, while the native structure of the TBEV E dimer is shown in gray. The two structures are aligned with EDIII, leading to the remaining domains being discrete. Both the top view and the side view of the structures are present in cartoon and transparent surface modes. Yellow arrows represent the possible squeezing and elevating of the conformational changes after DIII is associated with MAb 4.2 scFv. (B) The region within the white dashed line in panel A is shown in detail on the left side, while the right side represents the 7.3-Å elevation of the αB helix. The distance between two adjacent αB helices shifts from 8.8 Å to 4.2 Å upon antibody association. (C) Domain movements after binding to MAb 4.2 (not shown in this panel). DI and the fusion loop are elevated by 9.7 and 4.7 Å, respectively. The top view reveals that the fusion loop is squeezed 4.6 Å toward the dimerization interface.
