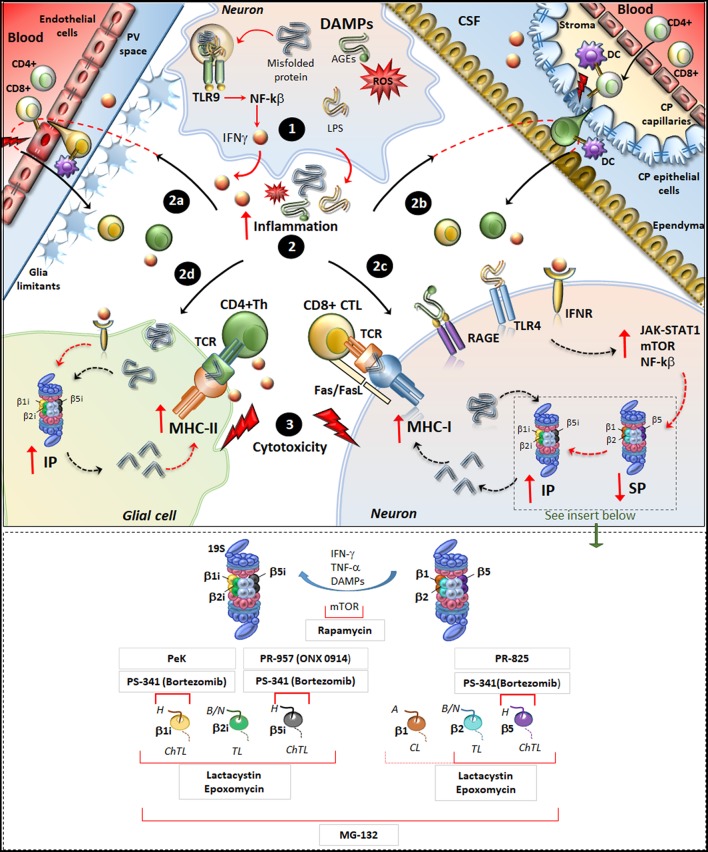
Figure 1.
Molecular mechanisms underlying IP induction in neurons and glia in neurodegenerative disorders. (Upper panel) Within neurons, an oxidative/inflammatory challenge or the presence of misfolded proteins leads to the production of DAMPs such as ROS, LPS, and AGEs. DAMPs bind to TLR9 to activate NF-kb and produce pro-inflammatory cytokines (1). DAMPs and misfolded/oxidized proteins and cytokines are then released extracellularly, which triggers an inflammatory reaction within the brain parenchyma (2). This fosters the recruitment of peripherally primed T-cells which are reactivated by APCs along the blood-brain barrier (2a) and blood-CFS barrier (2b), including DCs in the perivascular space (PV), in the choroid plexus (CP) stroma and CSF, as well as CP epithelial cells and endothelial cells of the brain-blood-barrier. In this way auto-reactive CD4+ T cells (green) and CD8+ T-cells (yellowish) recruit their effector machineries to damage CNS barriers (flashlights) and infiltrate the brain parenchyma. At the same time, misfolded/oxidized proteins, DAMPs and IFNs spread throughout the brain parenchyma and they bind to their receptors IFNr, RAGEs and TLR4 which are expressed in glia and neurons (2c, 2d). These activate common intracellular pathways namely JAK/STAT, NF-kβ, and mTOR, which downregulate/disassembly SP to foster induction and de-novo synthesis of IP. Thus, IP produces Ag peptides which bind to MHC-I molecules in neurons (2c) or even to MHC-II in glia (2d). MHC-antigen complexes are then transported to the cell surface to be presented to auto-reactive CD8+ CTLs and CD4+ Th lymphocytes, which trigger cytotoxicity and cytokine-mediated damage in neurons and glia (3). Figure Insert. Schematic overview of the mechanism of action of various IP/SP inhibitors listed in Table 1. On the right, the SP with its subunits β1, β2, and β5 which possess caspase-like (CL), trypsin-like (TL) and chymotrypsin-like (ChTL) activity, respectively. Following inflammatory/oxidative stimuli (IFN-γ, TNF-α, or DAMPs release), SP subunits are replaced with IP subunits and de-novo synthesis of IP occurs. On the left, IP with its subunits β1i, β2i and β5i which possess ChTL, TL, and ChTL activity, respectively. Rapamycin, mTOR inhibitor, reduces the synthesis of IP subunits and enhances P26S-dependent protein degradation; PeK (Peptidyl epoxyketone), selective epoxyketone of inhibitor of β1i; PS-341 (Bortezomib), reversible dipeptide boronate inhibitor of SP and IP with high affinity for β5, β5i, and β1i; PR-957 (also known as ONX 0914), irreversible β5i -selective epoxyketone inhibitor; PR-825, irreversible β5-specific inhibitor; Epoxomycin, irreversible and selective inhibitor of both SP and IP with high affinity for β2, β5, β1i, β2i, β5i; Lactacystin, similar to Epoxomycin; MG-132, nonspecific inhibitor of all β subunits of the 20S core particles within both SP and IP. H, hydrophobic; B/N, basic/neutral; A, acidic substrates.