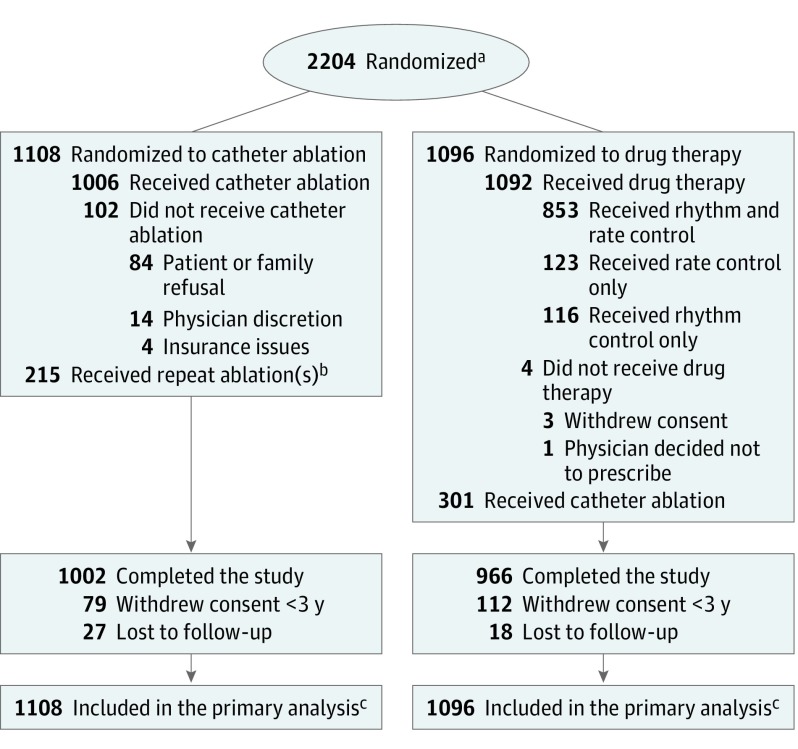
Figure 1. Randomization and Patient Flow in the CABANA Trial.
aSites were not required to provide screening logs during the recruitment phase; thus, the number of patients assessed for eligibility is not available.
bTwenty five patients underwent repeat catheter ablation during the blanking period; 190 patients had at least 1 repeat catheter ablation during the postblanking period for a total of 215.
cOutcomes of patients who did not complete the study (ie, withdrew consent or were lost to follow-up) were included to the point of consent withdrawal or final contact. Primary and key secondary end points were analyzed using time-to-event methodology; thus, all available follow-up information was used. For patients who did not complete the study and did not experience an outcome event, their time-to-event measure was censored at the last contact date. There was no imputation of outcome events. At the end of the trial, a publicly available death registry search was performed for patients enrolled in North America who were lost or withdrew from the trial.