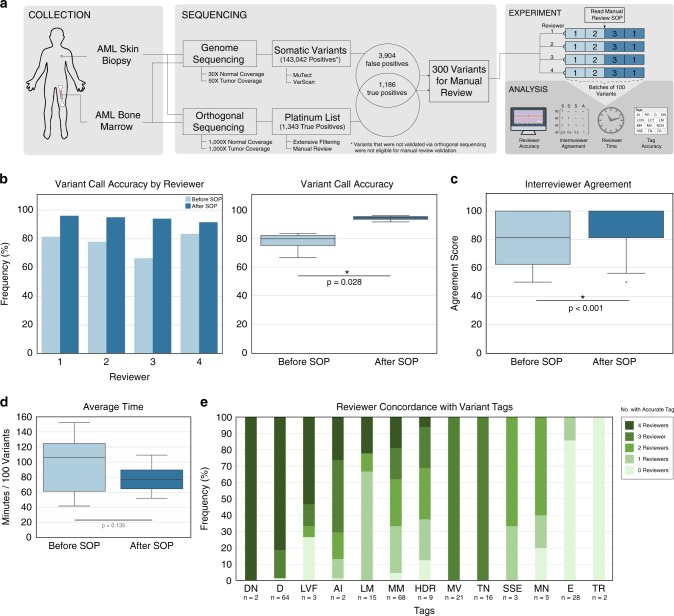
Fig. 4.
Validation of the manual review standard operating procedure (SOP). a Sequencing data from an acute myeloid leukemia (AML) case was used to test the impact of the SOP on accurately identifying somatic variants. A total of 300 variants that had genome sequencing and orthogonal sequencing were identified for the experiment. Four novice reviewers assessed 200 variants prior to and after reading the SOP to determine improvement in accuracy, reduction in interreviewer variability, change in reviewer time per variant, and appropriate use of tags. b Reviewer accuracy was assessed before and after reading the SOP. The bar plot shows accuracy stratified by reviewer and the box plot shows the reviewers’ cumulative median accuracy. c Box plot showing the median interreviewer agreement before and after reading the SOP. Agreement for each variant was calculated by assessing the correlation between the four reviewer calls using a correlation matrix as described in the Methods. d Box plot showing the median time required to conduct manual review before and after reading the SOP. e Frequency diagram showing the number of reviewers that correctly annotated false positive variants with gold standard tags, parsed by tag. AI Adjacent Indel, D Directional, DN Dinucleotide repeat, E End of reads, HDR High Discrepancy Region, LM Low Mapping, LVF Low Variant Frequency, MM Multiple Mismatches, MN Mononucleotide repeat, MV Multiple Variants, SSE Same Start End, TN Tumor in Normal, TR Tandem Repeat.