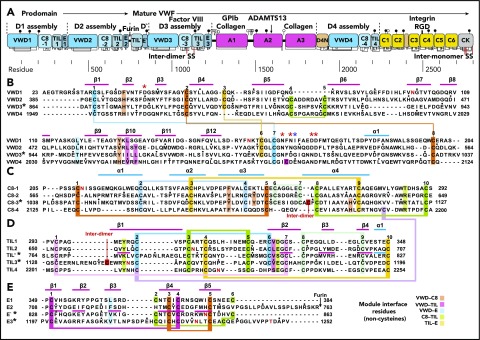
Figure 2.
Domain architecture of VWF and sequence of modules in D assemblies. (A) Domain architecture. Cysteines are vertical lines and are connected for disulfide bonds. N- and O-linked glycans are closed and open lollipops, respectively. Domains are scaled to length, and residues are shown with pre-pro numbering. (B-E) D assembly modules are aligned by sequence with insertions and deletions moved to loops between secondary structures defined here; in addition, the TIL′ and TIL3 modules and E′ and E3 modules are aligned by structure. Modules in the crystal structure are indicated with an asterisk. Disulfide-linked cysteines defined in the structure and the newly predicted disulfide between C8-4 and TIL4 are linked with colored lines. Module interface residues are highlighted as shown in the key in the lower right. α helices and β strands are shown in cyan and magenta lines, respectively, above sequence blocks for the D3 assembly or above TIL′ and E′ sequences for D′. Glycosylated Asn residues are shown in red. Ca2+-coordinating residues are asterisked in red (sidechain) or blue (backbone). Dots appear above decadal residues.