Figure 5.
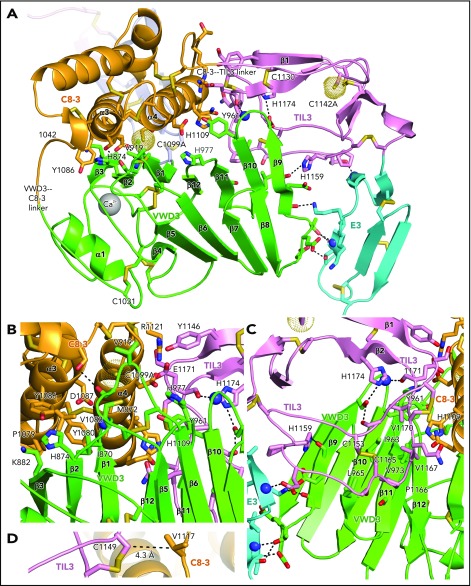
Intermodule interfaces in D3. (A) Overview. (B) View down the α3 and α4 helices of C8-3, emphasizing interfaces with VWD3 and TIL3. (C) View of TIL3 interfaces, with the view rotated ∼180° about the axis vertical in the page relative to A to show the opposite side of β strands 9 to 12. All residues that interact across module interfaces (with heavy atoms within 3.7 Å, excluding at intermodule peptide connections) are shown with sidechains and hydrogen-bonding backbone carbonyl groups in stick and nitrogens as spheres. Intermodule hydrogen bonds are shown as dashed lines. (D) Residues in D3 that align with cysteines in D4 and that have Cβ atom distances (dashed line) ideal for disulfide bond formation in D4 between the TIL4 and C8-4 modules. Important residues are labeled. Other representations are as in Figure 1.