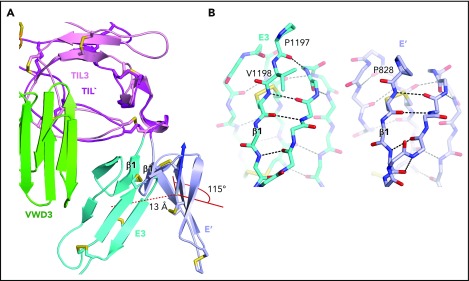
Figure 6.
An extra residue in E3 alters TIL-E module orientation. E3 has 1 more N-terminal residue than the other E domains in VWF (Figure 2E). (A) Superposition on TIL3 of TIL′ shows how TIL3- E3 orientation brings E3 much closer to VWD3 than would occur with the TIL′-E′ orientation. There is a 115° difference in orientation angle about the axis shown with the cone and cylinder and a 13-Å difference in E module center of mass. (B) E3 and E′ are shown in same orientation after superposition and horizontal separation on the page. The extra N-terminal residue in E3 extends the β1-β2 β ladder by 1 position and the rotation of the polypeptide backbone alters the orientation of P1197 relative to P828 and results in the 115° rotation at the TIL-E3 interface, as shown in panel A.