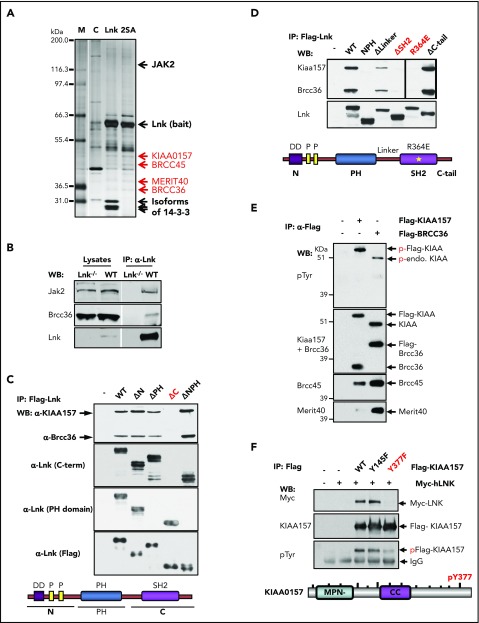
Figure 1.
The BRISC complex is a novel Lnk binding partner. (A) Identification of a novel Lnk–BRISC interaction. Cytoplasmic protein extracts of 32D-B/A parental cells (C) or 32D cells expressing Flag/HA-tagged WT Lnk or Lnk2SA mutant (2SA) deficient in 14-3-3 interaction, were immunoprecipitated with anti-FLAG and anti-HA antibodies sequentially. A small aliquot of precipitates was resolved in sodium dodecyl sulfate–polyacrylamide gel electrophoresis and protein bands visualized by silver stain as shown. Subsequently, a large aliquot of precipitates was stained by using Coomassie stains and protein bands identified by using mass spectrometry. Subunits of the BRISC complex are indicated in red. (B) Confirmation of Lnk–BRISC interaction. Lysates from WT and Lnk−/− spleens were either directly subjected to WB analysis (left) or precipitated with anti-Lnk antibodies followed by WB analysis (right) with indicated antibodies. (C) The Lnk C terminus binds to BRISC. Lysates from 32D-B/A cells stably expressing Flag-WT or mutant Lnk were precipitated with anti-Flag antibodies followed by WB analysis. The bottom illustrates the Lnk structure. N terminus (N) contains a dimerization domain and 2 proline-rich regions. (D) The Lnk SH2 domain binds to BRISC. 32D-B/A cells stably expressing Flag-WT Lnk or constructs containing mutations or small deletions in the C terminus of Lnk were generated. IP-WB analyses were performed as in panel C. The bottom illustrates the Lnk structure and indicates the mutated regions. *R364E point mutation. (E) KIAA0157 is tyrosine phosphorylated in hematopoietic cells. HEL cells expressing either Flag-tagged KIAA0157 or BRCC36 were precipitated with anti-Flag antibodies followed by WB analysis with antibodies to phospho-tyrosine (pTyr) and the BRISC components as indicated. (F) Lnk binds to pY377 in KIAA0157. HEL cells expressing Flag-KIAA0157 or mutants, along with Myc-Lnk, were lysed and precipitated with anti-Flag antibodies followed by WB analysis with the indicated antibodies. 4G10 antibodies recognize pTyr. The bottom illustrates the KIAA0157 structure. Δ, deletion; CC, coil-coiled domain that interacts with BRCC36; endo, endogenous; F, phenylalanine; FH, Flag/HA-tagged; M, marker; P, phosphorylated form; Y, tyrosine residues.