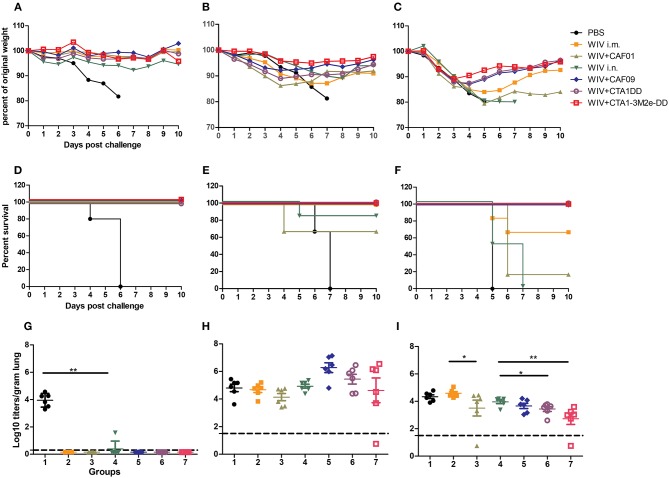
Figure 1.
Adjuvanted i.n. administered vaccine provides best protection. CB6F1 mice were vaccinated thrice 10 days apart with PBS, non-adjuvanted or adjuvanted WIV vaccines. Three weeks after the last vaccination, 6 mice/group were challenged with homologous PR8 (H1N1) (A,D,G), heterologous A/California/7/2009 (H1N1)pdm09 (B,E,H) or heterosubtypic X-31 (H3N2) viruses (C,F,I). Six animals/group were followed for 10 days for weight loss (A–C) and survival (D–F). Three days post challenge 6 mice/group were sacrificed for determining lung viral load (G–I). Groups are represented as in Table 1: 1: PBS, 2: WIV i.m., 3: WIV+CAF01 i.m., 4: WIV i.n., 5: WIV+ CAF09 i.n., 6: WIV+ CTA1-DD i.n., 7: WIV+ CTA1-3Me-DD i.n. Dashed line indicates Limit of detection (LoD) (G–I). Virus titers are represented as log10 titers/gram of lung tissue with level of significance as *p < 0.05 and **p < 0.01 calculated using Mann-Whitney U-test.