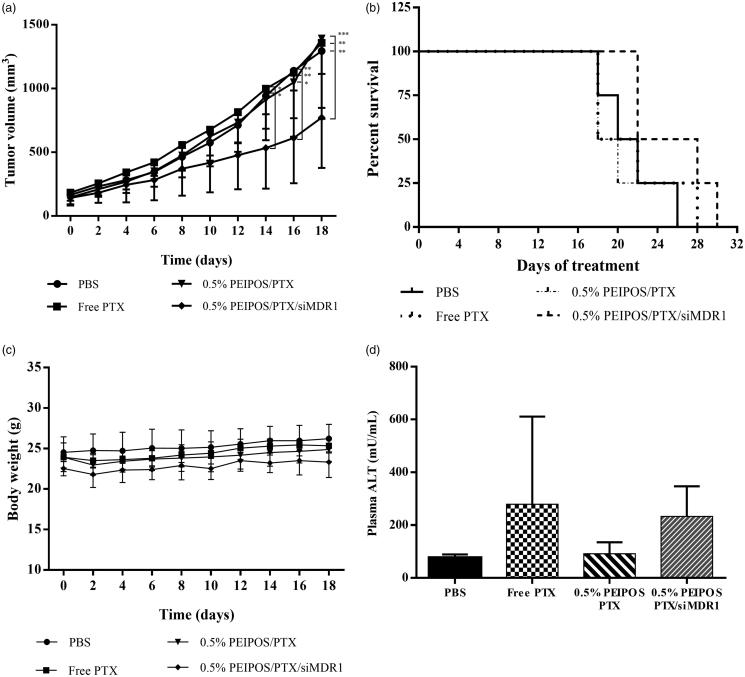
Figure 6.
In vivo evaluation of the formulation efficacy in nude athymic mice bearing A2780-ADR resistant human ovarian tumor xenografts. (a) Antitumor efficacy of liposomal and nonencapsulated PTX formulations on nude athymic mice bearing A2780-ADR resistant human ovarian tumor xenografts. After tumors were established, mice were treated every other day (first injection on day 0) with saline (•), PTX solubilized in Cremophor (?), 0.5%PEIPOS/PTX (?) or 0.5%PEIPOS/PTX/siMDR1(?) at 5.5 mg/kg of PTX and 0.8 mg/kg of siMDR1. (n = 4) Each point represents a mean ± S.D., two-way ANOVA with Dunnett’s multiple comparisons. ****p < .0001 (b) The survival curve of tumor-bearing animals. Mice were treated as indicated in (a) throughout the study period. The survival end-point was when tumors reached 1500 mm3. (c) No significant differences were observed in body weight of the animals. (d), Effect of different treatments on liver function evaluated by quantification of alanine aminotransferase (ALT) levels in blood samples collected from the animals before euthanasia. Only a mild increase in ALT levels in animals treated with 0.5% PEIPOS/PTX/siMDR1 was observed, but no statistical difference was identified among the groups.