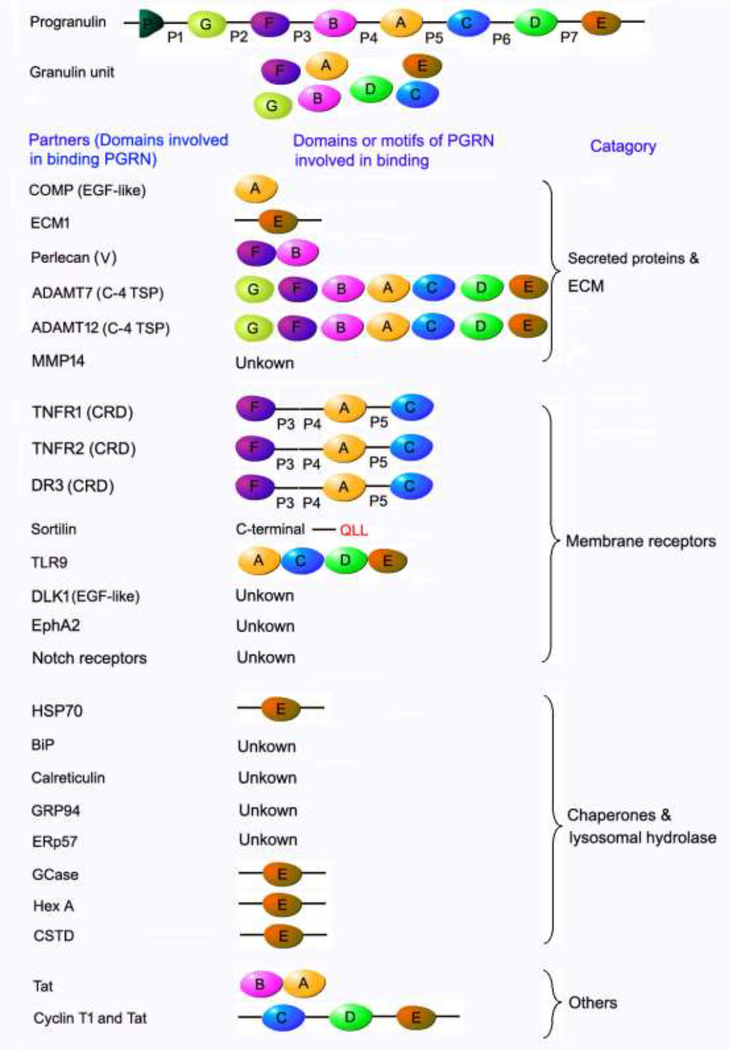
Figure 1. Summary of the domains of PGRN known to be involved in the interactions of PGRN with its binding partners.
PGRN possesses a high plasticity to bind to a wide spectrum of ECM proteins, membranous receptors and cytoplasmic chaperone and lysosomal hydrolases due to its unique beads-on-a-string structure and multiple binding domains. The domains known to be involved in PGRN and binding partner interactions are indicated. The cysteine rich domain (CRD) of TNFR and DR3 has been experimentally demonstrated to be required for interactions of these receptors with PGRN. Interestingly, a CRD is also present in the extracellular domains of Sortlin, EphA2, Notch receptors and TLR9. In addition, the extracellular domains of both Dlk1 and Notch receptors contain EGF-like domains, which is also known to bind to PGRN. It is expected that CRD and EGF-like domains are probably involved in the interactions of these aforementioned receptors with PGRN, although these associations need to be experimentally validated. “unknown” indicates that the binding domain(s) in PGRN remains to be determined.