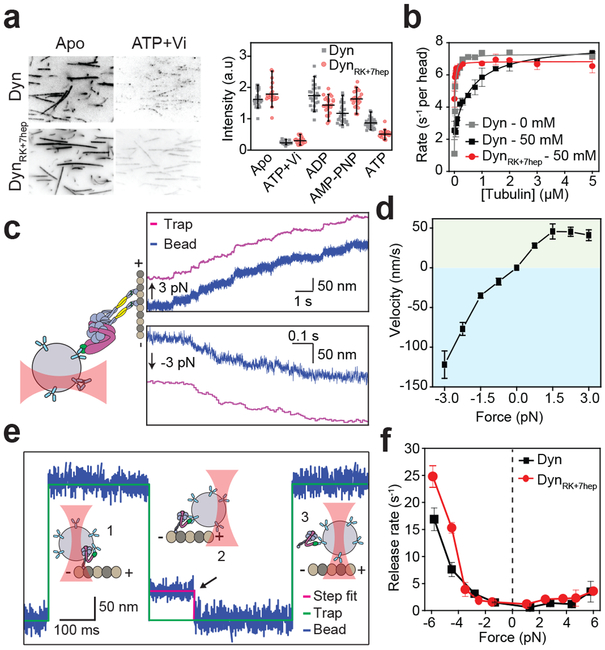
Figure 3 ∣. Reversal of the stalk angle does not disrupt nucleotide- and force-induced release of dynein from MT.
a, (Left) Representative images and (Right) the intensity of 100 nM GFP-tagged Dyn and DynRK+7hep monomers on sea urchin axonemes under given nucleotide conditions. Similar to Dyn, DynRK+7hep released from MTs in the ADP-Pi state, mimicked by ATP and vanadate (Vi, n = 40 axonemes from three independent measurements, mean ± 95% confidence intervals). b, MT-stimulated ATPase activity of dynein in 2 mM ATP (mean ± s.d. from three independent measurements) under different salt concentrations. Solid curves represent fit to the Michaelis-Menten kinetics. c, (Left) Full-length DynRK+7hep was attached to a polystyrene bead and pulled by constant load using optical trap (not to scale). (Right) Representative trajectories of single DynRK+7hep when pulled by 3 pN towards the plus- (positive forces, n = 20 beads) and minus-end (negative forces, n = 20 beads) of a MT. d, Force-velocity relationship of DynRK+7hep in the apo condition (mean ± s.e.m., n = 20, 21, 19, 16, 15, 23, 15, 15, 20 beads from left to right). e, Dynein monomers were attached to a polystyrene bead through their linker and oscillated ±150 nm along the MT long-axis by optical trap (1). When a molecule binds to the MT (2), the movement of the bead to the next trap position is restricted and the trap exerts a constant force until the motor releases from the MT (the black arrow, 3). f, Similar to Dyn, DynRK+7hep favors faster release from a MT when pulled towards the minus-end. Rates are calculated from an exponential decay fit to the dwell time histograms (n = 120 dwells for each force range from two biological replicates, mean ± s.d.).