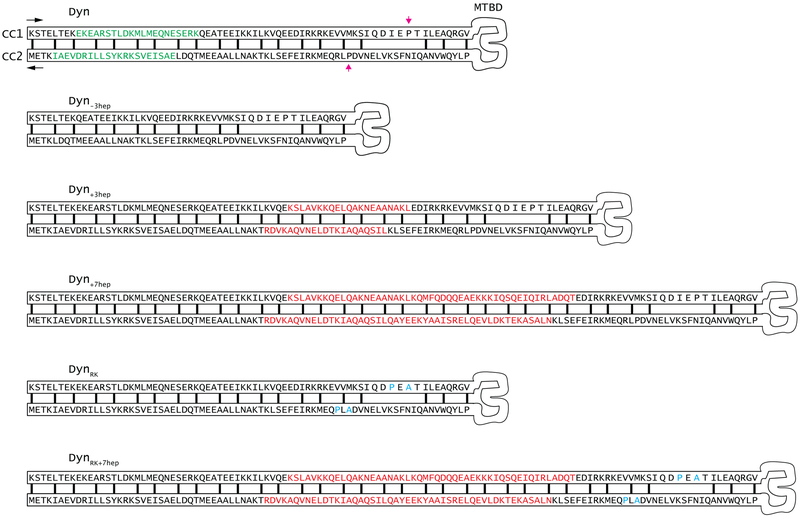
Extended Data Figure 2 ∣. Engineering the directionality of dynein motility.
Schematic diagram of the helices (CC1 and CC2) at the stalk of yeast cytoplasmic dynein shows the heptad repeat hydrophobic contacts (black lines) in the core of the coiled-coil when dynein is at low MT affinity (β) state. Conserved proline residues at the base of Dyn’s stalk are highlighted with magenta arrows. 3 heptads deleted from the stalk of Dyn−3hep are highlighted with green in Dyn. 3 and 7 heptad repeats inserted to Dyn+3hep, Dyn+7hep and DynRK+7hep are highlighted in red. The inserted sequences were taken from the Drosophila melanogaster cytoplasmic dynein12. Point mutations inserted to DynRK and DynRK+7hep are highlighted in cyan.