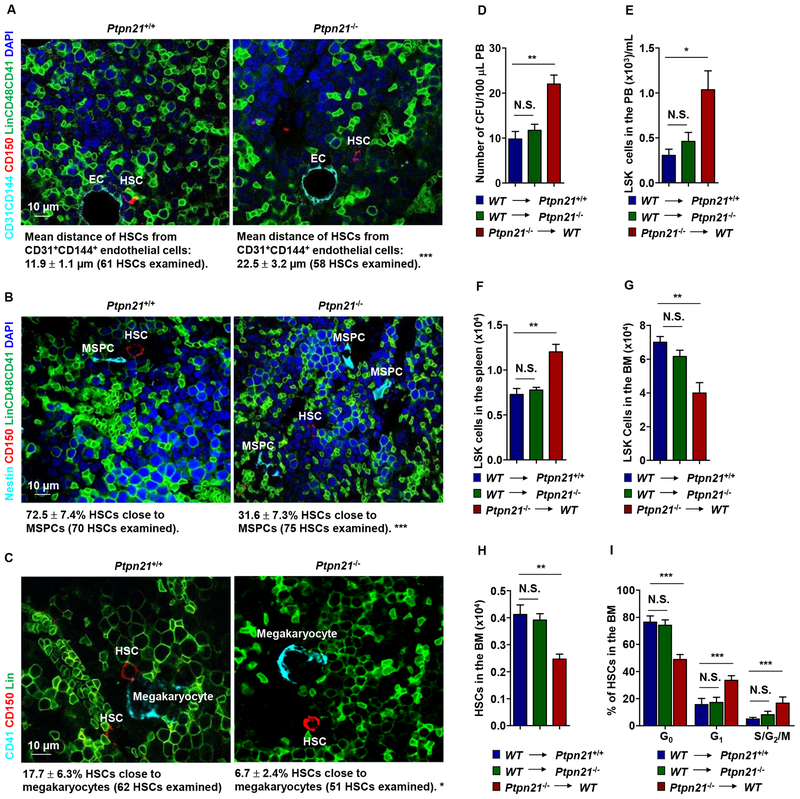
Figure 3. Deletion of Ptpn21 Results in HSC Dislocation and Stem/Progenitor Cell Egress in Ptpn21 Global Knock-out Mice through a Cell Intrinsic Mechanism.
Bone sections (one section per femur or tibia) prepared from 6–8 week-old Ptpn21−/− and Ptpn21+/+ mice were immunostained with the indicated antibodies. (A) The distance of HSCs (Lin–CD48–CD41–CD150+) from the closest CD31+CD144+ endothelial cells was determined (n = 5 mice per genotype). (B) The spatial relationship between HSCs (Lin−CD48−CD41−CD150+) and Nestin+ MSPCs was examined. HSCs within <8 μm of MSPCs were considered close to these cells (n = 4 mice per genotype). (C) The spatial relationship between HSCs (Lin−CD41−CD150+) and megakaryocytes (CD41+) was examined. HSCs within <8 μm of megakaryocytes were considered close to megakaryocytes (n = 4 mice per genotype). Representative images are shown. (D-I) BM cells collected from 3-month-old Ptpn21−/− mice (CD45.2+) were transplanted into lethally irradiated WT BoyJ mice (CD45.1+). In addition, BM cells collected from BoyJ mice were transplanted into 3-month-old Ptpn21−/− or Ptpn21+/+ mice (n = 5–7 per group). At 20 weeks following transplant, PB, spleen, and BM cells collected from the recipients were examined by CFU assays for hematopoietic progenitors (D) and FACS analyses for LSK cells and HSCs (E-H). In addition, the cycling status of HSCs in the BM were determined by FACS analyses (I).