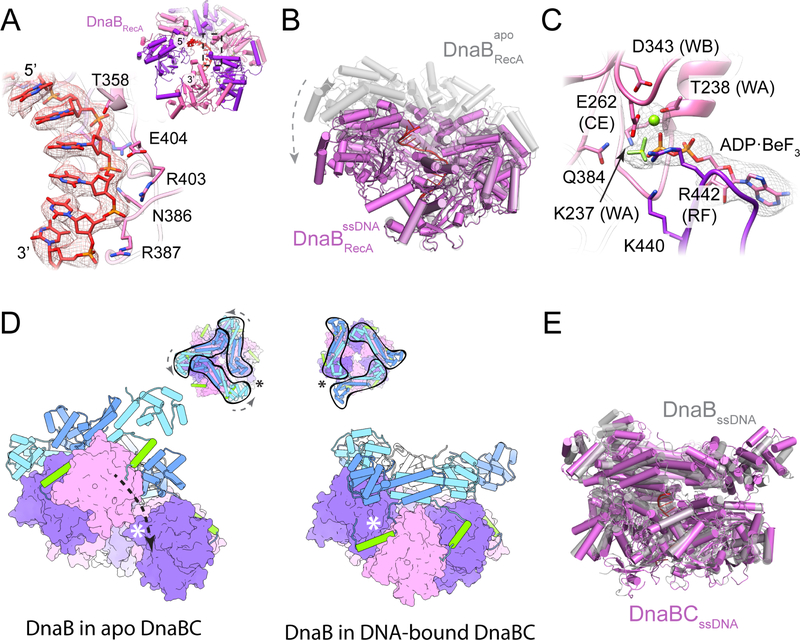
Figure 6. DNA remodels the molecular organization of DnaB.
(A) Close-up view DnaB residues involved in DNA binding. Density contoured at a threshold value of 0.05. (Inset) View of ssDNA bound to the RecA domains of DnaB.
(B) The DnaB RecA domains adopt a more planar, crack-ring configuration in the presence of nucleic acid.
(C) ADP•BeF3 is associated with the active sites of ssDNA-bound DnaB. Density contoured at a threshold value of 0.05.
(D) A terminal DnaB subunit relocates from one end of the cracked helicase ring to the other during a conformation transition induced by ssDNA binding. The open crack in the DnaB ring in absence of DNA is sealed off by the linker helix following DNA engagement, topologically linking the helicase around DNA. (Inset) Top-down views showing the conformational transition in the DnaB collar and the shift in the position of the crack in the helicase ring following the transition. Asterisks denote the position of the crack in the DnaB ring.
(E) The configuration of the helicase in the ssDNA-bound DnaBC complex is highly reminiscent to that seen for the free helicase bound to nucleic acid and GDP•AlFx (PDB 4ESV (Itsathitphaisarn et al., 2012)).