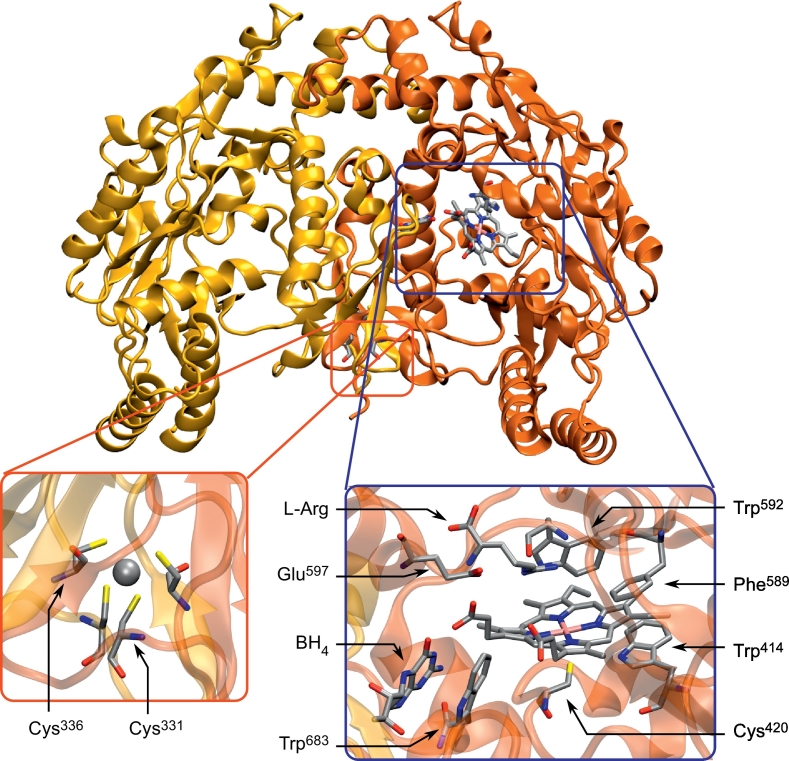
Fig. 3.
Structure of the human nNOSox dimer (PDB: 4D1N). Monomers are colored differently for sake of clarity. The left magnified section (red) shows the ZnS4 motif, with arrows pointing to the cysteines of the first monomer and the central zinc cation depicted in Van der Waals representation. The right magnified section (blue) provides a view of the active site, with the heme moiety in the center, coordinated to Cys420 on one side and dioxygen on the other. Interacting with the heme carboxylates, the pterin BH4 cofactor forms π-stacking interactions with Trp683. Glu597 stabilizes the substrate above the heme moiety, and the aromatic residues Phe589 and Trp414 sandwich the latter to maintain the proper organization of the active site. Trp592, presumably involved in the electron transfer from NOSred to NOSox, is located at the bottom of the heme-binding pocket. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)