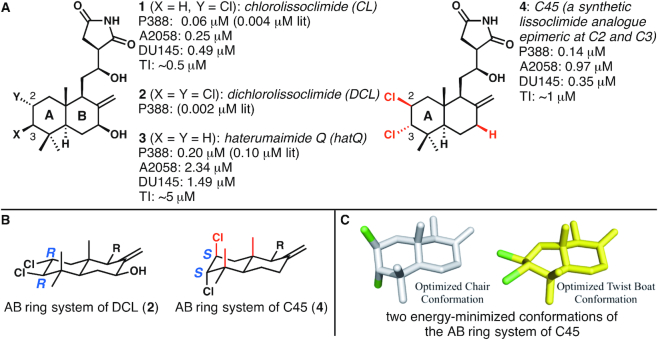
Figure 1.
The structures of natural lissoclimide compounds chlorolissoclimide, dichlorolissoclimide, haterumaimide Q and synthetic analogue C45. (A) All data shown are IC50 values against the cell lines shown (P388: murine leukemia; A2058: aggressive melanoma; DU145: aggressive prostate cancer), except for TI, which represents translation inhibition data. Literature values of IC50s against P388 (indicated by ‘lit’) are taken from reference (12), and all other data are taken from reference (3). (B) A comparison of the configurations of the C2 and C3 chloride-bearing stereogenic centers in the natural product DCL and the synthetic analogue C45. (C) Energy-minimized conformations of the AB ring system of C45: the first has the A ring in a chair conformation, and four substituents axial; the second has the A ring in a twist-boat conformation, thus relieving multiple 1,3-diaxial interactions.