Abstract
Aim
To examine the association of proton pump inhibitor (PPI) use with subsequent hip fracture incidence in hip fracture patients, accounting for gender, age, PPI doses, PPI initiation before or after first fracture, and year from first fracture in which the first subsequent fracture occurred.
Methods
Data from 31,668 Austrian patients ≥50 years with the first hip fracture between July 2008 and December 2010 were analyzed retrospectively. After exclusion of patients on anti-osteoporotic medication, incidence of subsequent hip fractures was compared between users and non-users of PPIs using regression models.
Results
In general, use of PPIs among hip fracture patients was associated with increased risk for subsequent hip fracture (OR 1.58, 95%-CI 1.25–2.00), in particular in men, in the age group of 70–84 years, and when PPIs were initiated before the first fracture. Low PPI doses of ≤90 cumulative DDDs and ≤0.25 DDDs/day, however, were not linked to elevated subsequent fracture risk, especially among female patients. Subsequent hip fracture incidence was elevated within the first year after first fracture in female and male PPI users (OR 1.75, 95%-CI 1.28–2.38) and dropped in women but not in men in the second year.
Conclusions
Low-dose PPI use is not associated with increased risk of subsequent hip fractures, especially in women. Patients thus get most benefit of short-term PPI use after a hip fracture that has previously been linked to lowered mortality if low doses are not exceeded. Varying risk profiles for the time of subsequent hip fracture could have implications for risk group-specific follow-up care.
Keywords: Hip fracture, Proton pump inhibitors, Subsequent fracture, Dosage, Osteoporosis
Highlights
-
•
PPI users were at particularly high risk of subsequent hip fracture within the first year after first fracture
-
•
Use of proton pump inhibitors (PPIs) was generally associated with increased risk of subsequent hip fractures
-
•
Low PPI doses ≤90 DDDs and ≤0.25 DDDs/day were not associated with increased subsequent hip fractures, particularly in women
-
•
Short-term PPI use at low doses has positive effects on survival after hip fracture as demonstrated recently
1. Introduction
Proton pump inhibitors (PPIs) are acid-suppressive drugs that are prescribed as co-medication particularly to elderly patients to prevent stress ulcers and/or detrimental drug-related side-effects on the gastro-intestinal tract. Also, PPIs are the mainstay for management and prophylaxis of stress ulcers in surgical patients, but there are no guidelines specifically for geriatric hip fracture patients (Thaler et al., 2013). While PPIs are considered as safe medication with which to efficiently treat e.g. peptic reflux, gastro-intestinal bleeding and ulcers, also adverse implications have been associated with long-term PPI use, including bone fractures (Malfertheiner et al., 2017). There are, however, controversial proposals on how PPIs might exert effects on bone. As a possible mechanism, malabsorption of calcium due to increased gastric pH has been suggested (O'Connell et al., 2005), but conversely, also inhibition of osteoclastic activity was reported (Mizunashi et al., 1993), potentially conveying a reduction in fracture risk. Furthermore, the observed rise in fracture risk associated with PPI use has been ascribed to residual confounding and selection bias, thus questioning a causative role of PPIs (Pouwels et al., 2011).
Osteoporosis, a systemic skeletal disease particularly affecting the elderly, is hallmarked by bone loss and deterioration of bone quality, finally resulting in fragility fractures (Sambrook and Cooper, 2006). Among osteoporotic fractures, hip fractures are the most severe type entailing a high burden of morbidity and mortality and health care expenses (Hernlund et al., 2013). Epidemiological evidence has linked use of PPIs with moderately increased risk of hip fracture incidence (Malfertheiner et al., 2017; Kwok et al., 2011; Ngamruengphong et al., 2011; Ye et al., 2011; Yu et al., 2011; Zhou et al., 2016), but also lack of risk has been reported (Malfertheiner et al., 2017; Kaye and Jick, 2008; Chen et al., 2016). Notably, findings of the relationship of duration and dosage of PPI use with hip fracture incidence are heterogeneous: With increasing duration, notions of elevated (Yang et al., 2006; Targownik et al., 2008), but also of unchanged risk or an inconsistent risk trend (Pouwels et al., 2011; Ngamruengphong et al., 2011; Ye et al., 2011; Zhou et al., 2016; Cea Soriano et al., 2014) have been put forth. Likewise, whereas a clear dose-response relationship was found in some investigations (Yang et al., 2006; Cea Soriano et al., 2014; Corley et al., 2010; Chiu et al., 2010), it was absent or vague in others (Pouwels et al., 2011; Ngamruengphong et al., 2011; Vestergaard et al., 2006).
It is well-established that prior fracture poses a risk factor for ensuing fractures, also at the hip (Kanis et al., 2004), and it has been shown that a fracture following a femur or femoral neck fracture is most likely to occur at the same site (Muschitz et al., 2017). No study as yet has, however, explicitly examined how PPIs might modify subsequent hip fracture risk. We therefore, in a nationwide retrospective cohort study, investigated into subsequent hip fracture incidence in Austrian hip fracture patients aged ≥50 years on PPIs vs. controls, excluding patients on anti-osteoporotic drugs, also taking into account varying cumulative and average PPI doses.
2. Methods
2.1. Study design and patient data
In this retrospective cohort study, we retrieved anonymized data on hip fracture patients from thirteen Austrian social insurance authorities encompassing approximately 98% of the entire population, using SAS 9.3 as database software (SAS Institute Inc., Cary, North Carolina). Based on unambiguous anonymous coding, multiple registrations due to re-admission could be ruled out. We thus identified 31,668 inpatients aged ≥50 years at hospital discharge who sustained the first hip fracture in the study interval, hence termed “index fracture”, between July 2008 and December 2010, with follow-up for survival and subsequent fractures until June 2011. The ICD-10 code classes of S72 were applied for identification of hip fractures. Hospitalization due to a hip fracture after the index fracture was considered as new hip fracture only when it occurred at least six months after the index fracture or a previous subsequent fracture, so as to exclude hospital stays for follow-up care due to the same fracture event (Y.K. Lee et al., 2013). Baseline characteristics of the study population (Brozek et al., 2014) and of PPI prescription data (Brozek et al., 2017) were described previously. The local Ethics Committee approved the study which was performed in agreement with the Declaration of Helsinki.
2.2. Outcome and covariates
Outcome was one or more subsequent hip fracture(s), and covariates were gender, age at index fracture, and follow-up time from index fracture.
2.3. Drug exposure
The WHO criteria (World Health Organisation Collaborating Centre for Drug Statistics Methodology, 2018) were applied for defining the amount of drug equivalent to one defined daily dose (DDD), i.e. 20 mg omeprazole, 40 mg pantoprazole, 30 mg lansoprazole, 20 mg rabeprazole, and 30 mg esomeprazole. For each patient, we recorded the sum of DDDs, i.e. cumulative DDDs, of prescriptions for proton pump inhibitors (PPIs) filled between July 2007 and June 2011. In addition, average DDDs were obtained by dividing cumulative DDDs by the number of days from first PPI prescription in the study interval to the end of follow-up (end of study or death, whichever occurred first). We decided to average cumulative DDDs of each patient over the time until end of follow-up because PPIs are often prescribed and/or taken on an irregular basis only when gastro-intestinal symptoms are present (Vestergaard et al., 2006). This means that the number of days of the treatment period beginning with the first and extending beyond the last prescription date is usually greater than the number of daily prescriptions. In contrast to the approach to divide cumulative DDDs by the number of daily prescriptions (Ye et al., 2011; Yang et al., 2006; de Vries et al., 2009), the present analysis takes into consideration treatment gaps.
2.4. Defining cohorts and matched cohorts analysis
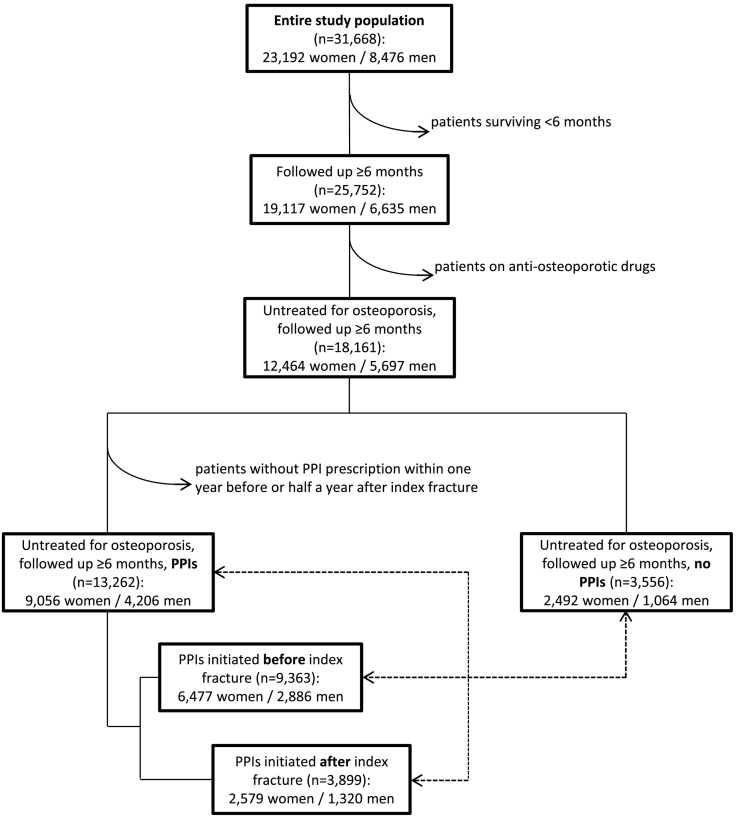
For studying effects of PPIs on subsequent hip fractures, we excluded patients not at risk for a subsequent fracture because they died within six months after the index fracture (i.e., follow-up time less than six months). We also excluded patients receiving anti-osteoporotic drugs filled between July 2007 and June 2011, including bisphosphonates, strontium ranelate, raloxifene, teriparatide, PTH, calcitonin, and denosumab, because of possible interference of those drugs with fracture risk (Fig. 1). Because of the effect on bone of such medications and interactions between PPIs and bisphosphonates that were predominantly used in our study population (Abrahamsen et al., 2011; J. Lee et al., 2013), results of our study targeting at effects of PPIs might otherwise be distorted. We thus identified 18,161 patients untreated for osteoporosis and followed up for at least six months who were divided into (i) controls who had never received PPIs throughout the study interval (n = 3556), and (ii) patients on PPIs with a prescription within one year before or not later than half a year after the index fracture (n = 13,262). The cohort of PPI users was further divided into two sub-groups according to the beginning of PPI prescription either before (n = 9363) or after (n = 3899) index fracture. All patients in the control group therefore survived at least the amount of time from index fracture until subsequent fractures could occur and until patients who began PPI treatment after index fracture had already started medication. Splitting up patients into PPI beginners before vs. after index fracture was motivated by previously obtained disparate results on mortality in these patient groups (Brozek et al., 2017). PPI users were compared with the control cohort using multivariate regression adjusting for gender, age at index fracture, and follow-up time. In addition, sub-cohorts of increasing PPI doses were matched with the control group by gender, age at index fracture, and follow-up time which was required to be within a range of ±10% of the corresponding patient on PPI treatment.
Fig. 1.
Study flow chart.
2.5. Statistical analysis
Binary logistic regression analysis informed on odds ratios (ORs) for incidence of at least one subsequent hip fracture. This method was preferred over time-dependent Cox proportional hazards regression analysis, because PPI doses were available only for the whole study period, thus time to event and drug doses could not be correlated. Life table analysis was used to monitor unadjusted cumulative mortalities and incidences of first subsequent hip fractures. In the matched cohorts analyses, the χ2 test served to compare the number of subsequent hip fractures between control and treatment groups, and the Mann-Whitney U test was used to verify that average follow-up time did not differ between matched cohorts. Applying a confidence level of 95%, differences were considered statistically significant at p < 0.05. All analyses were conducted in SPSS, version 19 (SPSS Inc., Chicago, Illinois).
3. Results
Baseline characteristics of the study population are shown in Table 1. When PPIs were initiated after index fracture, significantly more men than women were on PPIs and follow-up was longer, compared with non-users of PPIs. Median follow-up was 1.54 years (IQR 1.00–2.24) for all PPI users corresponding to 21,648.6 patient years, 1.52 years (IQR 0.98–2.18) for non-users of PPIs corresponding to 5703.8 patient years, 1.49 years (IQR 0.96–2.15) for PPI users who began medication pre-index fracture (14,787.6 patient years), and 1.73 years (IQR 1.14–2.41) for PPI users who began post-index fracture (6861.0 patient years). The composition of age groups differed significantly between non-users and users of PPIs, and there were significantly more subsequent hip fractures among PPI users than non-users. Supplemental Fig. 1 shows cumulative mortality and incidence of the first subsequent hip fracture up to two years after index fracture for users and non-users of PPIs, and for both genders separately. Mortality is high during the first six months, particularly in non-users, and PPIs users sustained more first subsequent hip fractures than non-users. Women, regardless whether on PPIs or not, had more first subsequent hip fractures than their male counterparts.
Table 1.
Baseline characteristics of the study population in users vs. no users of PPIs.
| All PPI users (n = 13,262) | p vs. no users | PPIs begun pre-index fracture (n = 9363) | p vs. no users | PPIs begun post-index fracture (n = 3899) | pvs. no users | No users of PPIs (n = 3556) | |
|---|---|---|---|---|---|---|---|
| Men, n (%) | 4206 (31.17) | <0.05 | 2886 (30.82) | 0.32 | 1320 (33.85) | <0.0001 | 1064 (29.92) |
| Mean age at index fracture (years) ± SD | 79.62 ± 5.43 | 0.50 | 79.94 ± 10.11 | 0.06 | 78.88 ± 10.84 | 0.05 | 79.13 ± 17.90 |
| Median follow-up time (years) (IQR) | 1.54 (1.00–2.24) | <0.05 | 1.49 (0.96–2.15) | 0.09 | 1.73 (1.14–2.41) | <0.0001 | 1.52 (0.98–2.18) |
| Age group, n (%) | <0.0001 | <0.0001 | <0.01 | ||||
| 50–69 years | 2494 (18.81) | 1651 (17.63) | 843 (21.62) | 767 (21.57) | |||
| 70–84 years | 6084 (45.88) | 4344 (46.40) | 1740 (44.63) | 1466 (41.23) | |||
| 85+ years | 4684 (35.32) | 3368 (35.97) | 1316 (33.75) | 1323 (37.20) | |||
| Patients with at least one subsequent hip fracture, n (%) | 495 (3.73) | <0.0001 | 351 (3.75) | <0.0001 | 144 (3.69) | <0.01 | 84 (2.36) |
| One subsequent hip fracture, n (%) | 421 (3.17) | 296 (3.16) | 125 (3.21) | 70 (1.97) | |||
| Two subsequent hip fractures, n (%) | 65 (0.49) | 48 (0.51) | 17 (0.44) | 14 (0.39) | |||
| Three subsequent hip fractures, n (%) | 7 (0.05) | 6 (0.06) | 1 (0.03) | – | |||
| Four subsequent hip fractures, n (%) | 2 (0.02) | 1 (0.01) | 1 (0.03) | – | |||
| Total number of subsequent hip fractures, n | 580 | <0.0001 | 414 | <0.0001 | 56 | <0.01 | 98 |
As also demonstrated in Table 2, use of PPIs was overall associated with elevated risk of sustaining at least one subsequent hip fracture (adjusted OR 1.58, 95%-CI 1.25–2.00, p < 0.001). Stratified by gender, risk was greater in male (adjusted OR 1.99, 95%-CI 1.19–3.31, p < 0.01) than in female (adjusted OR 1.47, 95%-CI 1.13–1.92, p < 0.01) PPI users. PPI medication initiated before the index fracture was associated with greater subsequent hip fracture risk than initiated post-index fracture (adjusted OR 1.65 vs. adjusted OR 1.47, respectively). Age group-wise, we found the highest risk in PPI users aged 70–84 years (adjusted OR 1.98, 98%-CI 1.32–2.97, p < 0.001).
Table 2.
Risk of at least one subsequent hip fracture (shfx) in the study interval expressed as crude and adjusted odds ratios (ORs). Stratification was by PPI initiation before vs. after index fracture (fx), increasing cumulative daily defined PPI doses, gender, and age group (only when patients were not stratified according to pre- or post-index fx initiation of PPIs). n, number of patients; DDDs, defined daily doses.
| n (PPI users/controls) | % patients with at least one shfx (PPI users/controls) | Crude OR (95%-CI) | p | Adjusted ORa (95%-CI) | p | ||
|---|---|---|---|---|---|---|---|
| PPIs begun pre-index fx and post-index fx | |||||||
| All DDDs | All | 13,262/3556 | 3.73%/2.36% | 1.60 (1.27–2.03) | <0.0001 | 1.58 (1.25–2.00) | <0.001 |
| Women | 9056/2492 | 4.00%/2.69% | 1.51 (1.16–1.96) | <0.01 | 1.47 (1.13–1.92) | <0.01 | |
| Men | 4206/1064 | 3.16%/1.60% | 2.01 (1.21–3.35) | <0.01 | 1.99 (1.19–3.31) | <0.01 | |
| 50–69 years | 2494/767 | 3.61%/2.61% | 1.40 (0.86–2.29) | 0.18 | 1.40 (0.85–2.29) | 0.18 | |
| 70–84 years | 6084/1466 | 3.71%/1.84% | 2.06 (1.37–3.08) | <0.001 | 1.98 (1.32–2.97) | <0.001 | |
| 85+ years | 4684/1323 | 3.82%/2.80% | 1.38 (0.96–1.98) | 0.08 | 1.38 (0.96–1.99) | 0.08 | |
| PPIs begun pre-index fx | |||||||
| All DDDs | All | 9363/3556 | 3.75%/2.36% | 1.61 (1.26–2.05) | <0.001 | 1.65 (1.29–2.10) | <0.0001 |
| Women | 6477/2492 | 4.00%/2.69% | 1.51 (1.15–1.98) | <0.01 | 1.53 (1.16–2.01) | <0.01 | |
| Men | 2886/1064 | 3.19%/1.60% | 2.03 (1.20–3.42) | <0.01 | 2.10 (1.24–3.55) | <0.01 | |
| ≤90 DDDs | All | 1467/3556 | 2.52%/2.36% | 1.07 (0.72–1.58) | 0.74 | 1.14 (0.77–1.69) | 0.51 |
| Women | 947/2492 | 2.32%/2.69% | 0.86 (0.53–1.40) | 0.55 | 0.91 (0.56–1.48) | 0.70 | |
| Men | 520/1064 | 2.88%/1.60% | 1.83 (0.91–3.69) | 0.09 | 1.92 (0.95–3.89) | 0.07 | |
| 91–365 DDDs | All | 2182/3556 | 3.25%/2.36% | 1.37 (1.00–1.89) | 0.05 | 1.47 (1.06–2.02) | <0.05 |
| Women | 1496/2492 | 3.68%/2.69% | 1.36 (0.95–1.95) | 0.09 | 1.43 (0.99–2.06) | 0.05 | |
| Men | 686/1064 | 2.33%/1.60% | 1.47 (0.74–2.93) | 0.27 | 1.59 (0.79–3.18) | 0.19 | |
| 366–730 DDDs | All | 2077/3556 | 3.95%/2.36% | 1.70 (1.25–2.31) | <0.001 | 1.71 (1.26–2.34) | <0.001 |
| Women | 1493/2492 | 4.09%/2.69% | 1.54 (1.08–2.19) | <0.05 | 1.56 (1.10–2.23) | <0.05 | |
| Men | 584/1064 | 3.60%/1.60% | 2.30 (1.20–4.39) | <0.05 | 2.32 (1.21–4.47) | <0.05 | |
| 731–1095 DDDs | All | 1570/3556 | 4.27%/2.36% | 1.84 (1.33–2.55) | <0.001 | 1.78 (1.28–2.47) | <0.001 |
| Women | 1095/2492 | 4.75%/2.69% | 1.80 (1.25–2.61) | <0.01 | 1.70 (1.17–2.46) | <0.01 | |
| Men | 475/1064 | 3.16%/1.60% | 2.01 (0.99–4.05) | 0.05 | 2.09 (1.02–4.25) | <0.05 | |
| >1095 DDDs | All | 2067/3556 | 4.50%/2.36% | 1.95 (1.44–2.63) | <0.0001 | 1.87 (1.38–2.53) | <0.0001 |
| Women | 1446/2492 | 4.70%/2.69% | 1.79 (1.27–2.52) | <0.001 | 1.72 (1.22–2.44) | <0.01 | |
| Men | 621/1064 | 4.03%/1.60% | 2.58 (1.38–4.82) | <0.01 | 2.45 (1.31–4.60) | <0.01 | |
| PPIs begun post-index fx | |||||||
| All DDDs | All | 3899/3556 | 3.69%/2.36% | 1.58 (1.21–2.08) | <0.001 | 1.47 (1.11–1.93) | <0.01 |
| Women | 2579/2492 | 4.00%/2.69% | 1.51 (1.10–2.06) | <0.05 | 1.36 (0.99–1.87) | 0.05 | |
| Men | 1320/1064 | 3.11%/1.60% | 1.97 (1.11–3.49) | <0.05 | 1.86 (1.05–3.31) | <0.05 | |
| ≤90 DDDs | All | 2057/3556 | 2.04%/2.36% | 0.86 (0.59–1.25) | 0.43 | 0.82 (0.56–1.20) | 0.32 |
| Women | 1286/2492 | 1.87%/2.69% | 0.69 (0.43–1.10) | 0.12 | 0.65 (0.40–1.05) | 0.08 | |
| Men | 771/1064 | 2.33%/1.60% | 1.47 (0.75–2.87) | 0.26 | 1.39 (0.71–2.72) | 0.34 | |
| 91–365 DDDs | All | 1134/3556 | 4.76%/2.36% | 2.07 (1.46–2.93) | <0.0001 | 2.10 (1.48–2.98) | <0.0001 |
| Women | 794/2492 | 5.29%/2.69% | 2.02 (1.36–3.00) | <0.001 | 2.06 (1.38–3.07) | <0.001 | |
| Men | 340/1064 | 3.53%/1.60% | 2.25 (1.06–4.76) | <0.05 | 2.30 (1.08–4.89) | <0.05 | |
| >365 DDDs | All | 708/3556 | 6.78%/2.36% | 3.01 (2.09–4.33) | <0.0001 | 2.18 (1.48–3.20) | <0.0001 |
| Women | 499/2492 | 7.41%/2.69% | 2.90 (1.92–4.38) | <0.0001 | 2.05 (1.33–3.16) | <0.01 | |
| Men | 209/1064 | 5.26%/1.60% | 3.42 (1.58–7.41) | <0.01 | 2.88 (1.23–6.76) | <0.05 | |
Adjusted for age at index fracture, follow-up time, and gender (except upon stratification for gender).
Whereas cumulative DDDs ≤90 were associated with no significantly greater subsequent hip fracture risk than in the control group, cumulative DDDs >90 entailed a significantly increased risk (e.g., adjusted OR 1.87, 95%-CI 1.38–2.53, p < 0.0001, for >1096 DDDs), cf. Table 2. Divided by gender, male patients´ ORs, unlike female patients´, were elevated also at DDDs ≤90 but failed to reach statistical significance at PPI initiation before and after index fracture. By age groups, a dose-response relationship between increasing cumulative PPI doses and subsequent hip fracture risk was most distinctive in patients aged 70–84 years (Supplemental Table 2). In patients aged 50–69 years and ≥85 years, risk did not increase further with higher cumulative PPI doses, or even dropped at the highest doses.
Matched cohorts analysis (Supplemental Table 1) confirmed and extended the results on increasing cumulative DDDs obtained from analyzing the unmatched cohorts. No statistically significant increase in number of subsequent hip fractures and of patients with subsequent hip fractures was observed in PPI users receiving ≤90 DDDs. At higher cumulative DDDs, either subsequent hip fractures/1000 patient years or ORs for at least one subsequent hip fracture were statistically significantly increased for both genders combined, except in patients receiving 548–730 DDDs and 913–1095 DDDs of PPIs begun pre-index fracture. A dose-response relationship could, however, be noted only for PPI initiation post-index fracture, which was true for both genders.
Stratified by average DDDs (Table 3), the lowest subsequent hip fracture risk was observed at the lowest average doses of ≤0.25/day, especially in female patients. Overall and for genders separately, a dose-response effect was seen up to 1 DDD/day which was abolished at average doses above. This effect was most pronounced in the age group of 70–84 years, but risk declined at average DDDs >1/day (Supplemental Table 3). Patients who began PPIs post-index fracture receiving >1 DDD/day were only very few, we thus included them in the group of >0.25 DDDs/day. A drop in ORs was also observed for these (not shown).
Table 3.
Risk of at least one subsequent hip fracture (shfx) in the study interval expressed as crude and adjusted odds ratios (ORs), stratified by PPI initiation before vs. after index fracture (fx), increasing average daily defined PPI doses, and gender. n, number of patients; DDDs, defined daily doses.
| n (PPI users/controls) | % patients with at least one shfx (PPI users/controls) | Crude OR (95%-CI) | p | Adjusted ORa (95%-CI) | p | ||
|---|---|---|---|---|---|---|---|
| PPIs begun pre-index fx | |||||||
| ≤0.25 DDDs/day | All | 2840/3556 | 3.13%/2.36% | 1.34 (0.99–1.81) | 0.06 | 1.35 (0.99–1.82) | 0.06 |
| Women | 1893/2492 | 3.33%/2.69% | 1.25 (0.88–1.77) | 0.22 | 1.23 (0.87–1.75) | 0.25 | |
| Men | 947/1064 | 2.75%/1.60% | 1.74 (0.94–3.22) | 0.08 | 1.77 (0.95–3.28) | 0.07 | |
| >0.25–0.50 DDDs/day | All | 1806/3556 | 3.71%/2.36% | 1.59 (1.15–2.21) | <0.01 | 1.62 (1.17–2.25) | <0.01 |
| Women | 1292/2492 | 4.18%/2.69% | 1.58 (1.10–2.27) | <0.05 | 1.61 (1.11–2.32) | <0.05 | |
| Men | 514/1064 | 2.53%/1.60% | 1.60 (0.77–3.31) | 0.21 | 1.65 (0.79–3.44) | 0.18 | |
| >0.50–0.75 DDDs/day | All | 1738/3556 | 4.14%/2.36% | 1.79 (1.30–2.46) | <0.001 | 1.80 (1.31–2.49) | <0.001 |
| Women | 1242/2492 | 4.27%/2.69% | 1.61 (1.12–2.33) | <0.05 | 1.62 (1.12–2.35) | <0.05 | |
| Men | 496/1064 | 3.83%/1.60% | 2.45 (1.62–4.76) | <0.01 | 2.53 (1.30–4.94) | <0.01 | |
| >0.75–1.00 DDDs/day | All | 1279/3556 | 5.08%/2.36% | 2.21 (1.59–3.08) | <0.0001 | 2.26 (1.62–3.15) | <0.0001 |
| Women | 874/2492 | 5.15%/2.69% | 1.96 (1.34–2.89) | <0.001 | 1.99 (1.35–2.94) | <0.001 | |
| Men | 405/1064 | 4.94%/1.60% | 3.20 (1.66–6.17) | <0.001 | 3.28 (1.68–6.41) | <0.001 | |
| >1.00 DDDs/day | All | 1700/3556 | 3.41%/2.36% | 1.46 (1.04–2.05) | <0.05 | 1.48 (1.05–2.08) | <0.05 |
| Women | 1176/2492 | 3.74%/2.69% | 1.41 (0.96–2.07) | 0.08 | 1.42 (0.96–2.09) | 0.08 | |
| Men | 524/1064 | 2.67%/1.60% | 1.69 (0.83–3.45) | 0.15 | 1.72 (0.84–3.54) | 0.14 | |
| PPIs begun post-index fx | |||||||
| ≤0.25 DDDs/day | All | 2247/3556 | 2.45%/2.36% | 1.04 (0.73–1.46) | 0.84 | 0.94 (0.66–1.34) | 0.73 |
| Women | 1433/2492 | 2.51%/2.69% | 0.93 (0.62–1.41) | 0.74 | 0.83 (0.54–1.25) | 0.37 | |
| Men | 814/1064 | 2.33%/1.60% | 1.47 (0.76–2.85) | 0.25 | 1.34 (0.69–2.63) | 0.39 | |
| >0.25 DDDs/day | All | 1652/3556 | 5.39%/2.36% | 2.35 (1.74–3.19) | <0.0001 | 2.25 (1.66–3.06) | <0.0001 |
| Women | 1146/2492 | 5.85%/2.69% | 2.25 (1.59–3.18) | <0.0001 | 2.14 (1.51–3.04) | <0.0001 | |
| Men | 506/1064 | 4.35%/1.60% | 2.80 (1.47–5.31) | <0.01 | 2.69 (1.40–5.16) | <0.01 | |
| >0.25–1.00 DDDs/day | All | 1233/3556 | 6.24%/2.36% | 2.75 (2.01–3.78) | <0.0001 | 2.62 (1.90–3.60) | <0.0001 |
| Women | 852/2492 | 6.92%/2.69% | 2.69 (1.88–3.86) | <0.0001 | 2.52 (1.75–3.62) | <0.0001 | |
| Men | 381/1064 | 4.72%/1.60% | 3.05 (1.56–5.98) | <0.01 | 2.97 (1.50–5.87) | <0.01 | |
| >1.00 DDDs/day | All | 419/3556 | 2.86%/2.36% | 1.22 (0.66–2.25) | 0.52 | 1.20 (0.64–2.22) | 0.57 |
| Women | 294/2492 | 2.72%/2.69% | 1.01 (0.48–2.13) | 0.97 | 1.01 (0.48–2.14) | 0.97 | |
| Men | 125/1064 | 3.20%/1.60% | 2.03 (0.67–6.14) | 0.21 | 1.95 (0.63–6.00) | 0.24 | |
Adjusted for age at index fracture, follow-up time, and gender (except upon stratification for gender).
We also examined the association of PPI use with risk of the first subsequent hip fracture, i.e. the second hip fracture, during the first and second year after the index hip fracture (Table 4), as well as the third year (not shown) that was generally characterized by small numbers of fracture events. Overall, ORs were the highest in the first and third year after the index fracture (adjusted OR 1.75, 95%-CI 1.28–2.38, p < 0.001, and adjusted OR 1.74, 95%-CI 0.68–4.46, p = 0.25, respectively) and only moderately elevated in the second year. Both female and male PPI users displayed statistically significantly increased risk for the second fracture within the first year (adjusted OR 1.63, 95%-CI 1.15–2.32, p < 0.01, and adjusted OR 2.17, 95%-CI 1.12–4.20, p < 0.05, respectively). Thereafter, ORs were highly but non-significantly increased among male patients in the second year, and among female patients in the third year. In PPI users aged 70–84, ORs were highest in the first year after index fracture (adjusted OR 2.42, 95%-CI 1.37–4.30, p < 0.01). Very similar figures were obtained when patients were separated by PPI initiation before or after index fracture and stratified by gender.
Table 4.
Risk of first subsequent hip fracture (shfx) associated with PPI use in the 1st, 2nd, and 3rd year post-index fracture expressed as crude and adjusted odds ratios (ORs), stratified by gender, age group, and PPI initiation before vs. after index fracture (fx). n, number of patients.
| 1st year post-index fracture |
2nd year post-index fracture |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
n (PPI users/CO) |
Crude OR (95%-CI) | p | Adjusted ORa (95%-CI) | p |
n (PPI users/CO) |
Crude OR (95%-CI) | p | Adjusted ORa (95%-CI) | p | |
| % (first shfx) | % (first shfx) | |||||||||
| PPIs begun pre-index fx and post-index fx | ||||||||||
| Both genders | 13,262/3556 | 1.76 (1.29–2.39) | <0.001 | 1.75 (1.28–2.38) | <0.001 | 9407/2519 | 1.31 (0.89–1.92) | 0.17 | 1.29 (0.88–1.89) | 0.20 |
| 2.30%/1.32% | 1.66%/1.27% | |||||||||
| Women | 9056/2492 | 1.65 (1.16–2.35) | <0.01 | 1.63 (1.15–2.32) | <0.01 | 6461/1760 | 1.14 (0.75–1.74) | 0.54 | 1.12 (0.73–1.71) | 0.60 |
| 2.43%/1.48% | 1.75%/1.53% | |||||||||
| Men | 4206/1064 | 2.17 (1.13–4.20) | <0.05 | 2.17 (1.12–4.20) | <0.05 | 2946/759 | 2.23 (0.88–5.66) | 0.09 | 2.19 (0.86–5.55) | 0.10 |
| 2.02%/0.94% | 1.46%/0.66% | |||||||||
| 50–69 years | 2494/767 | 1.45 (0.79–2.66) | 0.22 | 1.47 (0.80–2.69) | 0.21 | 1857/563 | 1.22 (0.49–2.99) | 0.67 | 1.21 (0.49–2.99) | 0.68 |
| 2.45%/1.69% | 1.29%/1.07% | |||||||||
| 70–84 years | 6084/1466 | 2.50 (1.41–4.43) | <0.01 | 2.42 (1.37–4.30) | <0.01 | 4371/1059 | 1.64 (0.87–3.10) | 0.13 | 1.59 (0.84–3.01) | 0.16 |
| 2.19%/0.89% | 1.69%/1.04% | |||||||||
| 85+ years | 4684/1323 | 1.50 (0.94–2.41) | 0.09 | 1.52 (0.95–2.43) | 0.08 | 3179/897 | 1.09 (0.62–1.94) | 0.76 | 1.09 (0.61–1.93) | 0.77 |
| 2.37%/1.59% | 1.82%/1.67% | |||||||||
| PPIs begun pre-index fx | ||||||||||
| Both genders | 9363/3556 | 1.81 (1.32–2.49) | <0.001 | 1.84 (1.34–2.52) | <0.001 | 6441/2519 | 1.33 (0.89–1.97) | 0.16 | 1.34 (0.90–1.99) | 0.15 |
| 2.37%/1.32% | 1.68%/1.27% | |||||||||
| Women | 6477/2492 | 1.70 (1.19–2.44) | <0.01 | 1.72 (1.20–2.46) | <0.01 | 4495/1760 | 1.15 (0.74–1.78) | 0.54 | 1.15 (0.74–1.79) | 0.53 |
| 2.50%/1.48% | 1.76%/1.53% | |||||||||
| Men | 2886/1064 | 2.24 (1.14–4.39) | <0.05 | 2.27 (1.16–4.45) | <0.05 | 1946/759 | 2.28 (0.88–5.92) | 0.09 | 2.33 (0.90–6.07) | 0.08 |
| 2.08%/0.94% | 1.49%/0.66% | |||||||||
| PPIs begun post-index fx | ||||||||||
| Both genders | 3899/3556 | 1.62 (1.13–2.33) | <0.01 | 1.58 (1.10–2.27) | <0.05 | 2966/2519 | 1.28 (0.81–2.01) | 0.29 | 1.25 (0.79–1.96) | 0.34 |
| 2.13%/1.32% | 1.62%/1.27% | |||||||||
| Women | 2579/2492 | 1.53 (1.01–2.31) | <0.05 | 1.46 (0.96–2.21) | 0.08 | 1966/1760 | 1.13 (0.68–1.88) | 0.64 | 1.08 (0.65–1.81) | 0.76 |
| 2.25%/1.48% | 1.73%/1.53% | |||||||||
| Men | 1320/1064 | 2.03 (0.97–4.26) | 0.06 | 2.03 (0.97–4.27) | 0.06 | 1000/759 | 2.14 (0.77–5.97) | 0.15 | 2.12 (0.76–5.93) | 0.15 |
| 1.89%/0.94% | 1.40%/0.66% | |||||||||
Adjusted for age at index fracture, follow-up time, and gender (except upon stratification for gender).
4. Discussion
In the present investigation, we examined the association of PPIs and different dosages thereof with subsequent hip fracture incidence among hip fracture patients. The relevance of subsequent hip fractures is underpinned by the previous notion that femur and femoral neck fractures, if not the first fractures, predominantly tend to be subsequent fractures of the same site (Muschitz et al., 2017): In patients aged 54–70 years with low-trauma fractures, a subsequent fracture in the same skeletal region occurred most frequently at the femoral neck compared with other skeletal sites. We also extend the notion of an association of PPI use with increased hip fracture incidence to subsequent hip fractures.
In our study, risk to sustain a subsequent hip fracture appeared elevated for (i) PPI users compared with non-users, (ii) male relative to female PPI users, (iii) PPI users aged 70–84 compared with younger and older patients on PPIs, and (iv) patients with pre-index fracture initiation of PPIs compared with those with post-index fracture initiation. Numerous investigations agree upon moderately increased hip fracture risk associated with PPI use. Also, the notion of a greater risk for men than women is borne out by some reports on hip fracture incidence (Pouwels et al., 2011; Yu et al., 2011) but finds itself at odds with others (Zhou et al., 2016; Yu et al., 2008). In the present study, we did not have access to information on medications other than anti-osteoporotic drugs, and on co-morbidities which often exert adverse effects on bone and predispose to fractures, encompassing drugs such as glucocorticoids, loop diuretics, and anti-depressants, as well as disorders like diabetes, chronic obstructive pulmonary disease, cerebrovascular disease, dementia, renal failure, HIV infection, and rheumatoid arthritis (Watts, 2017; Kanis et al., 2005; Reyes et al., 2014). Since PPIs are often prescribed alongside such medications and diseases, confounding must be considered a major factor underlying our results. In this regard, gender-related differences in co-medication prescribed along with PPIs and associated co-morbidities might account for male patients´ increased risk. Male hip fracture patients have indeed been shown to be sicker than their female counterparts in a number of studies (for review, cf. (Sterling, 2011)), a finding that might be particularly mirrored by PPI users. Similarly, such confounding could play a role to account for age group-related differences in subsequent hip fracture risk encountered herein. Even though morbidity rises with advanced age in general (Piccirillo et al., 2008; Salive, 2013), it drops for certain conditions at a very old age, e.g. diabetes >90 years (Piccirillo et al., 2008; Barbieri et al., 2001), arguably due to affected patients´ lowered life expectancy (Piccirillo et al., 2008). Given the importance of diabetes as risk factor for fragility fractures (Napoli et al., 2017) and the wide-spread use of PPIs among diabetes patients (Krishnan et al., 2013), a decreased proportion of hip fracture patients affected by this condition might thus contribute towards the observed risk reduction in the oldest age group. This assumption might also hold for other co-morbidities. Alternatively, co-morbidity might rise faster with age among PPI users relative to non-users, but at a very old age, also non-users might reach a high degree of morbidity, entailing a drop in the odds ratio. Finally, our finding that patients on PPIs already before their index fracture were usually at higher risk to sustain subsequent fractures than those only after their index fracture arguably reflects higher co-morbidity among the pre-index fracture beginners.
Cumulative and average PPI doses >90 DDDs and >0.25 DDDs/day tended to entail elevated risk, however, average DDDs above 1.00/day were associated with a risk reduction compared with lower doses of 0.75–1.00/day. In support of a dose-response effect, Chiu et al. (2010) identified the highest risk of hip fracture for >91 cumulative DDDs, and in stroke patients, cumulative DDDs >365 were associated with greater hip fracture risk than lower doses (Lin et al., 2018). Similarly, Yang et al. (2006) and de Vries et al. (2009) reported the highest hip fracture risk at the highest average daily PPI doses. Conversely, Vestergaard et al. (2006) found the lowest odds ratio for hip fracture at cumulative and average PPI doses ≥100 DDDs and ≥1 DDD/day, respectively, without dose-response relationship, even upon adjustment for multiple factors. Their finding of low hip fracture risk for average DDDs ≥1 DDD/day compared with lower dosages is in line with our results. In our study, this finding can in part be explained by a disproportionately high number of PPI users with short follow-up thus decreasing the time in which subsequent fractures could occur in this dosage category. However, this applied only to patients with PPI initiation post-index fracture of whom 43 out of 419, i.e. 10.3%, used PPIs for less than half a year (data not shown). Finally, no statistically significant difference was detected between low-dose and high-dose PPI users in a previous meta-analysis (Ngamruengphong et al., 2011). These mixed results might thus also be mirrored by our findings.
The present study might not be able to resolve whether PPI medication plays a causative role in the risk of subsequent hip fractures, but we found that prescription of ≤90 cumulative DDDs of PPIs plus being on ≤0.25 DDDs/day is a necessary condition for not being at increased risk. If ORs >1.3 are (arbitrarily) defined to denote increased risk independent of the p-value, being a female hip fracture patient plus receiving ≤90 cumulative DDDs of PPIs plus being on ≤0.25 DDDs/day is both necessary and sufficient for being not at increased risk of subsequent hip fractures. Notably, this would be independent of pre- or post-index fracture initiation of PPIs and true of all age groups where the increase in ORs is driven by males. We have previously shown that PPI use in hip fracture patients, in particular during hospital stay and after discharge, significantly decreases short-term mortality up to 90 days post-hip fracture (Brozek et al., 2017). It is conceivable that targeted short-term administration of PPIs after a hip fracture can be achieved without exceeding low doses, thus minimizing subsequent hip fracture risk especially in women, besides exerting a beneficial effect on patient survival.
A major osteoporotic fracture like a hip fracture increases the risk of sustaining a subsequent major osteoporotic fracture (Kanis et al., 2004; Johansson et al., 2017), and this risk is particularly pronounced within the first two years following the first fracture (Johansson et al., 2017). In fact, we identified different risk profiles for female and male PPI users and across age groups with respect to when the first subsequent hip fracture occurred. It can be hypothesized that these patterns reflect gender- and age-related differences in co-morbidity. Therefore, male PPI users´ higher risk during the first two years after the index event might be based on their worse health status compared with female PPI users. By contrast, many of the presumably less sick female PPI users might be prone to their first subsequent fracture only during the end of the study period. The assumption that co-morbidity rises with advanced age but decreases at a very old age could account for the high risk in PPI users aged 70–84 years already in the first year. In this analysis, the number of subsequent fracture events declined disproportionately in relation to the decrease in number of patients at risk in each year from the first to the third year post-index fracture. Statistical significances were observed only in the first year after index fracture, so we paid less attention to the p-values in this analysis in favor of ORs alone.
There are limitations that characterize our study: Information on co-morbidities and co-medications except for anti-osteoporotic drugs was not available, thus no causal link between PPI use and risk of subsequent hip fractures can be established. Many of the findings are best explained by selection bias and confounding, including PPI-related differences in subsequent hip fracture risk between pre- and post-index fracture beginners and between genders. Also, high short-term mortality in the first six months following index fracture particularly among non-users of PPIs might have resulted in a selection of healthier patients less prone to subsequent fractures. However, longer survival increases the likelihood for PPI prescription, thus a disproportionately large number of patients who died soon after their index fracture were classified as non-users. Higher short-term mortality among non-users vs. users of PPIs hence might not necessarily reflect a selection process. Next, hospitalization within 6 months after a hip fracture was treated as follow-up care, hence any real subsequent fracture during this time would have been overlooked. Also, in order to make firm claims for long-term effects of PPIs the study period was definitely too short: For example, the number of fracture cases declined steeply towards the end of the study interval and 95%-CIs widened accordingly. Furthermore, information on over-the-counter (OTC) drugs was lacking, however, except for low dose omeprazole and pantoprazole at small packaging sizes (7 to 14 units) at high out-of-pocket expenses, in Austria PPIs are available for a very low prescription charge which rules out cost benefits from OTC medicines. Since prescription data have proven to be a reliable source in previous studies despite OTC availability of some of the drugs (Yood et al., 2007; Lam et al., 2013), we deem OTC drug use unlikely to be an important confounding factor. Finally, we consider as one advantage of our investigation that only issued prescriptions that were filled were accounted for; although filling a prescription is no guarantee of actually taking the drug, it has been demonstrated that the assessment of drug adherence based on filled prescriptions is a valid approach, which indicates that patients with a filled prescription usually also take the drug (Sattler et al., 2013). Furthermore, matching by follow-up time in the matched cohorts analysis so to rule out competing risk of mortality and immortal time bias should be considered as another strength of our study.
Collectively, we have shown that the use of PPIs among hip fracture patients is generally associated with increased risk of sustaining at least one subsequent hip fracture, except for low PPI doses, i.e. ≤90 DDDs and ≤0.25 DDDs/day among female patients in particular. Since we have recently demonstrated that PPI use entails reduction of short-term (90-day) mortality in hip fracture patients (Brozek et al., 2017), targeted short-term use of PPIs that does not exceed low doses, besides exerting beneficial effects on survival, is safe with respect to subsequent hip fracture risk, especially in women. However, it must be borne in mind that our results portend selection bias and confounding as the main sources of heterogeneity. Finally, given the high prevalence of PPI use among hip fracture patients, the identification of a gender- and age group-related risk profile among PPI users according to when after the index fracture the first subsequent fractures accumulated could have implications for targeted follow-up care for high risk patient groups.
Declarations of interest
None; Wolfgang Brozek, Berthold Reichardt, Jochen Zwerina, Hans Peter Dimai, Klaus Klaushofer, and Elisabeth Zwettler declare that they have no disclosures and no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Transparency document
Transparency document.
Acknowledgments
We thank the members of the Pharmacoeconomics Advisory Council of the Austrian Sickness Funds for provision of the data.
Footnotes
The Transparency document associated with this article can be found, in online version.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bonr.2019.100204.
Appendix A. Supplementary data
Supplementary material
References
- Abrahamsen B., Eiken P., Eastell R. Proton pump inhibitor use and the antifracture efficacy of alendronate. Arch Intern Med. 2011;171:998–1004. doi: 10.1001/archinternmed.2011.20. [DOI] [PubMed] [Google Scholar]
- Barbieri M., Rizzo M.R., Manzella D., Paolisso G. Age-related insulin resistance: is it an obligatory finding? The lesson from healthy centenarians. Diabetes Metab Res Rev. 2001;17:19–26. doi: 10.1002/dmrr.178. [DOI] [PubMed] [Google Scholar]
- Brozek W., Reichardt B., Kimberger O., Zwerina J., Dimai H.P., Kritsch D., Klaushofer K., Zwettler E. Mortality after hip fracture in Austria 2008–2011. Calcif Tissue Int. 2014;95:257–266. doi: 10.1007/s00223-014-9889-9. [DOI] [PubMed] [Google Scholar]
- Brozek W., Reichardt B., Zwerina J., Dimai H.P., Klaushofer K., Zwettler E. Use of proton pump inhibitors and mortality after hip fracture in a nationwide study. Osteoporos Int. 2017;28:1587–1595. doi: 10.1007/s00198-017-3910-x. [DOI] [PubMed] [Google Scholar]
- Cea Soriano L., Ruigómez A., Johansson S., García Rodríguez L.A. Study of the association between hip fracture and acid-suppressive drug use in a UK primary care setting. Pharmacotherapy. 2014;34:570–581. doi: 10.1002/phar.1410. [DOI] [PubMed] [Google Scholar]
- Chen C.-H., Lin C.-L., Kao C.-H. Gastroesophageal reflux disease with proton pump inhibitor use is associated with an increased risk of osteoporosis: a nationwide population-based analysis. Osteoporos Int. 2016;27:2117–2126. doi: 10.1007/s00198-016-3510-1. [DOI] [PubMed] [Google Scholar]
- Chiu H.-F., Huang Y.-W., Chang C.-C., Yang C.-Y. Use of proton pump inhibitors increased the risk of hip fracture: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2010;19:1131–1136. doi: 10.1002/pds.2026. [DOI] [PubMed] [Google Scholar]
- Corley D.A., Kubo A., Zhao W., Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139:93–101. doi: 10.1053/j.gastro.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernlund E., Svedbom A., Ivergård M., Compston J., Cooper C., Stenmark J., McCloskey E.V., Jönsson B., Kanis J.A. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H., Siggeirsdóttir K., Harvey N.C., Odén A., Gudnason V., McCloskey E., Sigurdsson G., Kanis J.A. Imminent risk of fracture after fracture. Osteoporos Int. 2017;28:775–780. doi: 10.1007/s00198-016-3868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman, Tennenhouse A (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed]
- Kanis J.A., Borgstrom F., De Laet C., Johansson H., Johnell O., Jonsson B., Oden A., Zethraeus N., Pfleger B., Khaltaev N. Assessment of fracture risk. Osteoporos Int. 2005;16:581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- Kaye J.A., Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy. 2008;28:951–959. doi: 10.1592/phco.28.8.951. [DOI] [PubMed] [Google Scholar]
- Krishnan B., Babu S., Walker J., Walker A.B., Pappachan J.M. Gastrointestinal complications of diabetes mellitus. World J Diabetes. 2013;4:51–63. doi: 10.4239/wjd.v4.i3.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok C.S., Yeong J.K.-Y., Loke Y.K. Meta-analysis: risk of fractures with acid-suppressing medication. Bone. 2011;48:768–776. doi: 10.1016/j.bone.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Lam J.R., Schneider J.L., Zhao W., Corley D.A. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435–2442. doi: 10.1001/jama.2013.280490. [DOI] [PubMed] [Google Scholar]
- Lee Y.-K., Ha Y.-C., Choi H.J., Jang S., Park C., Lim Y.-T., Shin C.S. Bisphosphonate use and subsequent hip fracture in South Korea. Osteoporos Int. 2013;24:2887–2892. doi: 10.1007/s00198-013-2395-5. [DOI] [PubMed] [Google Scholar]
- Lee J., Youn K.E., Choi N.-K., Lee J.-H., Kang D.Y., Song H.-J., Park B.-J. A population-based case-control study: proton pump inhibition and risk of hip fracture by use of bisphosphonate. J Gastroenterol. 2013;48:1016–1022. doi: 10.1007/s00535-012-0722-9. [DOI] [PubMed] [Google Scholar]
- Lin S.-M., Yang S.-H., Liang C.-C., Huang H.-K. Proton pump inhibitor use and the risk of osteoporosis and fracture in stroke patients: a population-based cohort study. Osteoporos Int. 2018;29:153–162. doi: 10.1007/s00198-017-4262-2. [DOI] [PubMed] [Google Scholar]
- Malfertheiner P., Kandulski A., Venerito M. Proton-pump inhibitors: understanding the complications and risks. Nat Rev Gastroentero Hepatol. 2017;14:697–710. doi: 10.1038/nrgastro.2017.117. [DOI] [PubMed] [Google Scholar]
- Mizunashi K., Furukawa Y., Katano K., Abe K. Effect of omeprazole, an inhibitor of H+,K(+)-ATPase, on bone resorption in humans. Calcif Tissue Int. 1993;53:21–25. doi: 10.1007/BF01352010. [DOI] [PubMed] [Google Scholar]
- Muschitz C., Kocijan R., Baierl A., Dormann R., Feichtinger X., Haschka J., Szivak M., Muschitz G.K., Schanda J., Pietschmann P., Resch H., Dimai H.P. Preceding and subsequent high- and low-trauma fracture patterns—a 13-year epidemiological study in females and males in Austria. Osteoporos Int. 2017;28:1609–1618. doi: 10.1007/s00198-017-3925-3. [DOI] [PubMed] [Google Scholar]
- Napoli N., Chandran M., Pierroz D.D., Abrahamsen B., Schwartz A.V., Ferrari S.L. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13:208–219. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- Ngamruengphong S., Leontiadis G.I., Radhi S., Dentino A., Nugent K. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209–1218. doi: 10.1038/ajg.2011.113. [DOI] [PubMed] [Google Scholar]
- O'Connell M.B., Madden D.M., Murray A.M., Heaney R.P., Kerzner L.J. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med. 2005;118:778–781. doi: 10.1016/j.amjmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Piccirillo J.F., Vlahiotis A., Barrett L.B., Flood K.L., Spitznagel E.L., Steyerberg E.W. The changing prevalence of comorbidity across the age spectrum. Crit Rev Oncol Hematol. 2008;67:124–132. doi: 10.1016/j.critrevonc.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels S., Lalmohamed A., Souverein P., Cooper C., Veldt B.J., Leufkens H.G., de Boer A., van Staa T., de Vries F. Use of proton pump inhibitors and risk of hip/femur fracture: a population-based case-control study. Osteoporos Int. 2011;22:903–910. doi: 10.1007/s00198-010-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes C., Estrada P., Nogués X., Orozco P., Cooper C., Díez-Pérez A., Formiga F., González-Macías J., Prieto-Alhambra D. The impact of common co-morbidities (as measured by the Charlson index) on hip fracture risk in elderly men: a population-based cohort study. Osteoporos Int. 2014;25:1751–1758. doi: 10.1007/s00198-014-2682-9. [DOI] [PubMed] [Google Scholar]
- Salive M.E. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. doi: 10.1093/epirev/mxs009. [DOI] [PubMed] [Google Scholar]
- Sambrook P., Cooper C. Osteoporosis. Lancet. 2006;367:2010–2018. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- Sattler E.L.P., Lee J.S., Perri M., 3rd Medication (re)fill adherence measures derived from pharmacy claims data in older Americans: a review of the literature. Drugs Aging. 2013;30:383–399. doi: 10.1007/s40266-013-0074-z. [DOI] [PubMed] [Google Scholar]
- Sterling R.S. Gender and race/ethnicity differences in hip fracture incidence, morbidity, mortality, and function. Clin Orthop Relat Res. 2011;469:1913–1918. doi: 10.1007/s11999-010-1736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targownik L.E., Lix L.M., Metge C.J., Prior H.J., Leung S., Leslie W.D. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMJA. 2008;179:319–326. doi: 10.1503/cmaj.071330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler H.W., Dovjak P., Iglseder B., Pinter G., Müller E., Müller W., Pils K., Mikosch P., Gerstorfer I., Zmaritz M., Weissenberger-Leduc M., Gosch M. Stress ulcer prophylaxis, thromboprophylaxis and coagulation management in patients with hip fractures. Wien Med Wochenschr. 2013;163:442–447. doi: 10.1007/s10354-013-0234-0. [DOI] [PubMed] [Google Scholar]
- Vestergaard P., Rejnmark L., Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79:76–83. doi: 10.1007/s00223-006-0021-7. [DOI] [PubMed] [Google Scholar]
- de Vries F., Cooper A.L., Cockle S.M., van Staa T.-P., Cooper C. Fracture risk in patients receiving acid-suppressant medication alone and in combination with bisphosphonates. Osteoporos Int. 2009;20:1989–1998. doi: 10.1007/s00198-009-0891-4. [DOI] [PubMed] [Google Scholar]
- Watts N.B. Adverse bone effects of medications used to treat non-skeletal disorders. Osteoporos Int. 2017;28:2741–2746. doi: 10.1007/s00198-017-4171-4. [DOI] [PubMed] [Google Scholar]
- World Health Organisation Collaborating Centre for Drug Statistics Methodology. https://www.whocc.no/atc_ddd_index/. Accessed 17 January 2018.
- Yang Y.-X., Lewis J.D., Epstein S., Metz D.C. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- Ye X., Liu H., Wu C., Qin Y., Zang J., Gao Q., Zhang X., He J. Proton pump inhibitors therapy and risk of hip fracture: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:794–800. doi: 10.1097/MEG.0b013e328348a56a. [DOI] [PubMed] [Google Scholar]
- Yood M.U., Campbell U.B., Rothman K.J., Jick S.S., Lang J., Wells K.E., Jick H., Johnson C.C. Using prescription claims data for drugs available over-the-counter (OTC) Pharmacoepidemiol Drug Saf. 2007;16:961–968. doi: 10.1002/pds.1454. [DOI] [PubMed] [Google Scholar]
- Yu E.W., Blackwell T., Ensrud K.E., Hillier T.A., Lane N.E., Orwoll E., Bauer D.C. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int. 2008;83:251–259. doi: 10.1007/s00223-008-9170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.E., Bauer S.R., Bain P.A., Bauer D.C. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124:519–526. doi: 10.1016/j.amjmed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Huang Y., Li H., Sun W., Liu J. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int. 2016;27:339–347. doi: 10.1007/s00198-015-3365-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.
Supplementary material