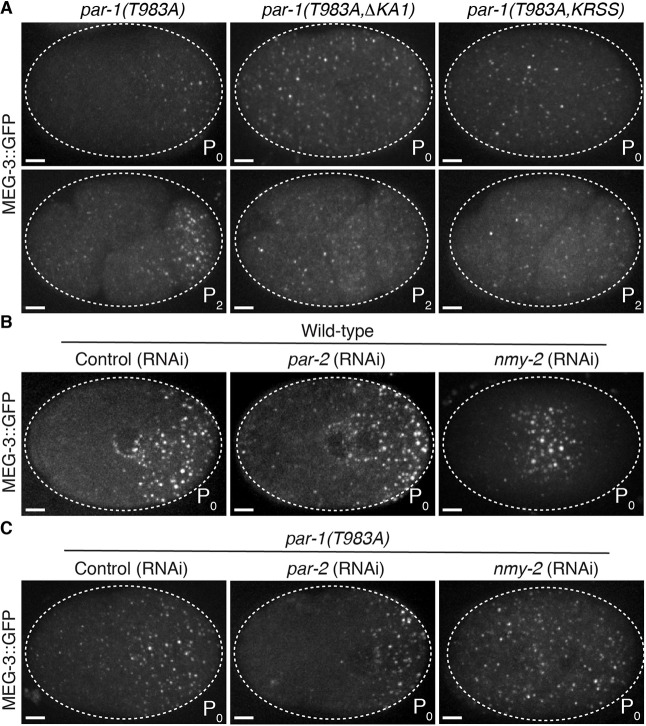
Fig. 7.
PKC-3 functions redundantly with KA1 and NMY-2 to inhibit PAR-1 activity in the anterior. (A) Photomicrographs of live par-1(T983A), par-1(T983A KRSS) or par-1(T983A ΔKA1) zygotes (top row) and four-cell embryos (bottom row) with GFP-tagged MEG-3. In par-1(T983A KRSS) or par-1(T983A ΔKA1) embryos, P granules assemble throughout the cytoplasm and are not asymmetrically segregated. (B,C) Photomicrographs of GFP-tagged MEG-3 in live wild-type (B) or par-1(T983A) (C) zygotes depleted for par-2 or nmy-2 by RNAi. Depletion of nmy-2, but not par-2, eliminates P granule asymmetry. In nmy-2 (RNAi), PKC-3 is enriched at the cortex throughout the zygote (Rodriguez et al., 2017) and thus inhibits P granule assembly at the periphery, unless T983 is mutated. Scale bars: 5 μm.