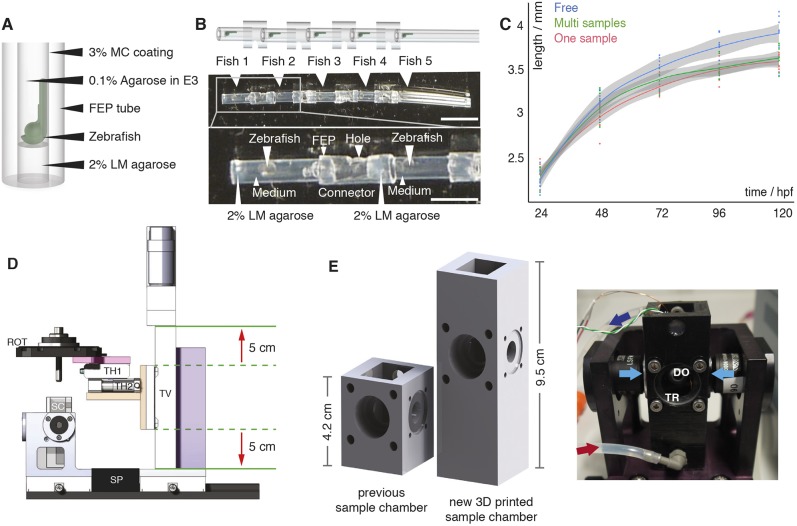
Fig. 1.
Multi-sample embedding and necessary microscope modifications. (A) Schematic of single zebrafish embryo embedding in a fluorinated polypropylene (FEP) tube using 2% low melting point (LM) agarose as a plug, 0.1% agarose in E3 as the medium and 3% methylcellulose (MC) for coating the FEP tube, as described previously (Kaufmann et al., 2012). (B) Schematic (top) and images (middle) of five mounted zebrafish (white arrowheads) in FEP tube pieces assembled using FEP connectors. The bottom image shows details of the embedding: zebrafish mounted in the FEP tubes rested on a 2% LM agarose plug and were embedded in E3 medium containing 0.1% agarose. FEP tubes containing a single zebrafish embryo were attached with FEP connectors containing a hole. Scale bar: 1 cm (top image); 4 mm (bottom image). (C) Growth curve of freely swimming fish (blue, n=8), individually embedded samples (red, n=10) and samples embedded in the multi-sample tube (green, n=9) with the 0.95% confidence interval of Loess interpolation in grey. (D) The new translational stage design of the multi-sample imaging platform ensured a vertical travel range of 10 cm (red arrows) and was built with custom-made parts, e.g. an adapter (pink) between the rotational stage (ROT) and the translational stage platform, an adapter (orange) between the horizontal translational stages (TH1 and TH2) and the vertical translational stage (TV), and a stage mount (purple). SP, spacer; SC, sample chamber. (E) Comparison of a traditional one-sample SPIM chamber with the new 3D-printed sample chamber and a picture of the new sample chamber connected to a perfusion system with inflow (red arrow) and outflow (dark blue arrow), the two illumination objectives for dual-sided illumination (light-blue arrows), a window for transmission (TR), and a detection objective (DO) in the back.