ABSTRACT
Vector-borne diseases cause over 700,000 deaths annually and represent 17% of all infectious illnesses worldwide. This public health menace highlights the importance of understanding how arthropod vectors, microbes and their mammalian hosts interact. Currently, an emphasis of the scientific enterprise is at the vector–host interface where human pathogens are acquired and transmitted. At this spatial junction, arthropod effector molecules are secreted, enabling microbial pathogenesis and disease. Extracellular vesicles manipulate signaling networks by carrying proteins, lipids, carbohydrates and regulatory nucleic acids. Therefore, they are well positioned to aid in cell-to-cell communication and mediate molecular interactions. This Review briefly discusses exosome and microvesicle biogenesis, their cargo, and the role that nanovesicles play during pathogen spread, host colonization and disease pathogenesis. We then focus on the role of extracellular vesicles in dictating microbial pathogenesis and host immunity during transmission of vector-borne pathogens.
KEY WORDS: Extracellular vesicle, Arthropod-borne disease, Cell communication, Immunomodulation, Microbial transmission
Summary: Extracellular vesicles mediate intra- and inter-kingdom cellular communication. This Review examines the emerging theme of extracellular vesicles directing arthropod physiology, microbial pathogenesis and host immunity.
Introduction
Vector-borne illnesses contribute to the global burden of human maladies, affecting hundreds of millions of people each year (WHO, 2017). Recently, there has been a significant increase in research dedicated to the understanding of arthropods as vectors of diseases because these ailments disproportionately impact vulnerable populations around the world. Mosquitoes, ticks and sandflies, among other arthropods, secrete salivary proteins that contribute to microbial transmission (Leitner et al., 2013; Shaw et al., 2016; Šimo et al., 2017). One immune evasion strategy used by vector-borne pathogens to promote a successful infection is through the secretion of extracellular vesicles (Atayde et al., 2015; Babatunde et al., 2018; Lovo-Martins et al., 2018; Nogueira et al., 2015; Silverman et al., 2010a,b; Sisquella et al., 2017; Trocoli Torrecilhas et al., 2009). Extracellular vesicles are involved in the exchange of molecular cargo between cells. They are present in the blood, lymph, saliva and urine, and have been investigated for their use as disease biomarkers (Cheshomi and Matin, 2018; McCarthy et al., 2016; Nair et al., 2018; Zhang et al., 2018).
Infected host cells and eukaryotic pathogens secrete vesicles containing antigens, nucleic acids and other microbial determinants that exacerbate pathogenesis and modulate host immunity (Fleming et al., 2014). The cargo within these vesicles may influence how the host responds to a pathogen, and how microbes communicate with each other (Eliaz et al., 2017). In this Review, we discuss our current understanding surrounding extracellular vesicle biogenesis in the context of vector-borne illnesses. We present the most up-to-date classification of extracellular vesicles, as well as general mechanisms of action in infectious diseases. Finally, we provide specific examples of how vesicles influence vector-transmitted infections, and how this knowledge may steer the future of arthropod vector research.
Biogenesis and classification of extracellular vesicles
Extracellular vesicles are heterogeneous and may be classified according to their biogenesis, content and biochemical features (Théry et al., 2009). Classically, they have been categorized as microvesicles and exosomes. Herein, we will briefly discuss biogenesis, characteristics, cargo and the re-classification of exosomes as a group. For a complete review of exosome and microvesicle biogenesis, please refer to van Niel et al., (2018). Importantly, it should be noted that the mechanisms discussed here have been investigated predominantly in mammalian systems, accentuating the limited understanding of extracellular vesicle biogenesis in arthropod vectors.
Exosomes
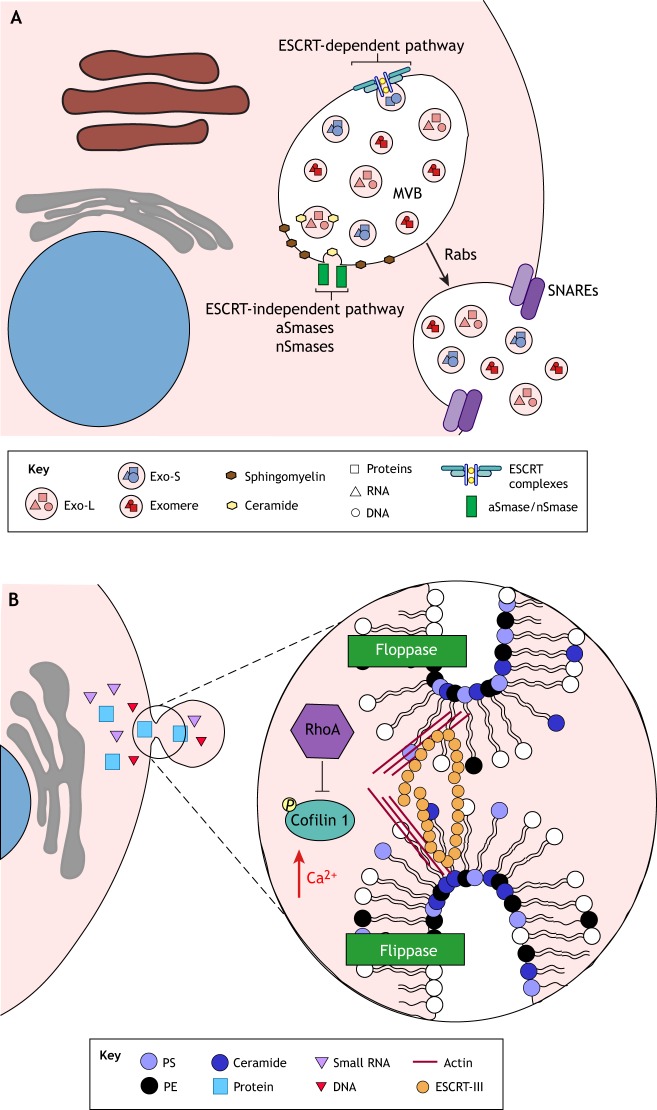
Exosomes are vesicles derived from multivesicular bodies (MVBs) (van Niel et al., 2018). These vesicles are formed by two different pathways: the endosomal sorting complex required for transport (ESCRT)-dependent and the ESCRT-independent network (Fig. 1A). In the ESCRT-dependent pathway, ESCRT complexes (ESCRT-0, -I, -II and -III) are involved in the budding of the membrane internally into the MVB (Baietti et al., 2012; Colombo et al., 2013; Sahu et al., 2011; Tamai et al., 2010). The ESCRT-independent pathway involves the hydrolysis of sphingomyelin into ceramide at the MVB membrane, which is mediated by the action of sphingomyelinases, also resulting in the budding of exosomes internally into the MVB (Trajkovic et al., 2008). Transport, tethering and docking of the MVB to the plasma membrane are mediated by Rab GTPases, a large family of Ras-like small GTPases that are associated with vesicular trafficking (Ostrowski et al., 2010). Once trafficked to the plasma membrane, the MVB fuses with the plasma membrane, possibly through the action of soluble N-ethylmaleimide-sensitive factor activating protein receptors (SNAREs) (Fader et al., 2009; Gross et al., 2012; Wei et al., 2017). At this juncture, exosomes are released into the extracellular space.
Fig. 1.
Exosome and microvesicle biogenesis. (A) Exosomes are formed in the lumen of multivesicular bodies (MVBs) through two biological signaling cascades: (1) the endosomal sorting complex required for transport (ESCRT)-dependent, and (2) the ESCRT-independent pathways. In the ESCRT-dependent pathway, exosomes are produced by the action of four different ESCRT complexes (ESCRT-0, -I, -II, and III). In the ESCRT-independent pathway, exosomes are created by the accumulation of ceramide when acid sphingomyelinases (aSmases) and neutral sphingomyelinases (nSmases) hydrolyze sphingomyelin. Rab proteins mediate the transport of the MVB, and SNARE molecules drive the fusion of vesicles with the plasma membrane. Fusion of membranes leads to the secretion of different subpopulations of vesicles (e.g. Exo-L, Exo-S and exomeres), which exhibit distinct DNA, RNA and protein signatures. (B) Microvesicles are secreted through the invagination of the plasma membrane. Flippases and floppases rearrange the lipid content of the outer layer of the plasma membrane, resulting in the enrichment of phosphatidylserine (PS), phosphatidylethanolamine (PE) and ceramide. Lipid remodeling changes the curvature of the plasma membrane, thereby forming the budding of the microvesicle, which is triggered by increases in Ca2+ concentration. The exact mechanism of microvesicle excision remains mostly unknown. However, the ESCRT-III complex and changes in actin dynamics driven by the small GTPase RhoA and cofilin 1 may be involved.
Exosomes contain specific cargo, including the molecules CD63, flotillin-1 and flotillin-2, G proteins and peroxidases (van Niel et al., 2018). They also carry lipids, DNA and RNA (van Niel et al., 2018). Sorting of proteins into exosomes appears to be partly regulated by ESCRT-dependent and ESCRT-independent mechanisms (van Niel et al., 2018). However, not much is understood about this process. Similarly, it remains elusive how the sorting of the genomic cargo into these nanovesicles occurs. Several of the micro (mi)RNAs identified in exosomes are not always detected in donor cells (Lunavat et al., 2015), indicating that specific small RNAs may be selected for packing into exosomes. Indeed, the sumoylated protein heterogeneous nuclear ribonucleoprotein (hnRNP) A2B1 (SUMO–hnRNPA2B1) and synaptotagmin-binding cytoplasmic RNA-interacting protein (SYNCRIP) specifically recognize so-called ‘Exo motifs’ in miRNAs for their sorting into exosomes (Santangelo et al., 2016; Villarroya-Beltri et al., 2013). Similarly, Y-box protein 1 (YBX1) specifically recognizes exosomal miRNAs for sorting into vesicles; however, no Exo motifs that YBX1 binds to have been identified to date (Shurtleff et al., 2016).
Exosomes are commonly categorized as being within the size range of 50 to 150 nm (van Niel et al., 2018). Importantly, their classification is currently evolving, and recent studies have identified distinct subpopulations (Willms et al., 2016; Zhang et al., 2018). We will focus on the recent description of exosomal sub-populations as proposed by Zhang et al. (2018).
Exo-S and Exo-L
A recent study has divided ‘exosomes’ into two distinct subpopulations based on their size: (i) Exo-S, which range from 60 to 80 nm, and (ii) Exo-L, which span between 90 and 120 nm in size (Zhang et al., 2018). Both Exo-S and Exo-L exosomes appear to be enriched for membrane- and ESCRT-associated proteins, integrins and Rabs. Proteins involved in G-protein and STAT signaling are overrepresented in Exo-L subpopulations. By contrast, Exo-S vesicles contain molecules that are often detected in the endosome (Zhang et al., 2018). The authors suggested that Exo-S represent ‘bona fide exosomes’ and proposed the use of flotillin-1 and flotillin-2 as markers for their identification. The lipid content of Exo-L and Exo-S nanovesicles varies depending on the cell of origin. Both subpopulations are enriched for small RNAs, miRNAs and tRNAs; however, only Exo-L vesicles contain a RNA peak at 315 base pairs, which could represent a population of small RNAs that is unique to this vesicle type. Exo-S and Exo-L are enriched with DNA between 2 kb to 4 kb. Finally, Exo-L vesicles have been described to be the only subpopulation that show a tropism for lymph nodes, indicating that this type of nanovesicles may facilitate tumor dissemination in mammalian systems (Zhang et al., 2018).
Exomeres
Exomeres are a recently described type of vesicle that lack an external membrane and have sizes smaller than 50 nm (Zhang et al., 2018). As such, the knowledge about these small vesicles remains obscure. They are enriched in proteins involved in metabolism, cytoskeleton and glycolysis. A potential protein marker for this subpopulation is the heat shock protein (Hsp90)-β. Exomeres have been described to carry higher DNA content, but fewer lipids and less RNA material when compared to Exo-S and Exo-L vesicles (Zhang et al., 2018).
Microvesicles
Microvesicle secretion is stimulated by the accumulation of Ca2+ in the cytoplasm and is a product of direct budding of the plasma membrane (Sedgwick and D'Souza-Schorey, 2018; Théry et al., 2009) (Fig. 1B). Flippases and floppases use Ca2+ to reorganize lipids within the cell membrane, inducing membrane budding through the accumulation of ceramide, phosphatidylserine (PS) and phosphatidylethanolamine (PE) in the outer layer (Beer et al., 2018; Théry et al., 2009; van Niel et al., 2018) (Fig. 1B). In addition to lipid rearrangements, cytoskeleton dynamics appear to play a role in the shedding of microvesicles. In cancer cells, microvesicle shedding is triggered by the small GTPase RhoA, which leads to the phosphorylation of cofilin 1 (Li et al., 2012). The ESCRT-III protein charged multivesicular body protein 4B (CHMP4B) appears to be associated with microvesicle release from cardiomyocytes (Xu et al., 2017). In addition, the tumor susceptibility gene (TSG101), an ESCRT-I protein, and the vacuolar protein sorting-associated protein 4 (VPS4, which has VPS4A and VPS4B forms in mammals), an ESCRT-III associated molecule, have also been implicated in microvesicle budding (Choi et al., 2018; Nabhan et al., 2012).
Microvesicles are commonly described as being within the range of 100 nm to 1 µm in size (Théry et al., 2009). Microvesicles contain proteins, lipids and genetic material, which are delivered to recipient cells. Their cargo is selectively sorted by specific proteins and depend on the secreting cell (van Niel et al., 2018). For example, vesicle-associated membrane protein 3 (VAMP-3) is involved in the packaging of membrane-type 1 matrix metalloprotease (MT1-MMP; also known as MMP14) into microvesicles (Clancy et al., 2015). Likewise, microvesicles have been shown to contain selectively sorted RNA cargo, including the enrichment of mRNAs containing a zip code sequence of 25 nucleotides in microvesicles (Bolukbasi et al., 2012). These findings suggest that cells may recognize and target certain mRNAs for packaging. Thus, microvesicles are thought to be involved in infectious diseases (Clancy et al., 2015; Hoshino et al., 2015; Moroishi et al., 2016).
Extracellular vesicles in infectious diseases
Extracellular vesicles have an important role in the establishment of infectious diseases (Schorey et al., 2015). As this area of research is an emerging field, we choose to focus on specific models and relate general concepts of microbial invasion, pathogenesis, host immunity and immune evasion. For more information, please refer to the following reviews: Altan-Bonnet (2016); Marti and Johnson (2016); Raab-Traub and Dittmer (2017); and Schorey and Harding (2016).
Immune activation
Extracellular vesicles are involved in immune activation and antigen presentation during Mycobacterium tuberculosis infection. M. tuberculosis manipulates the secretion of extracellular vesicles in mammalian cells by increasing the abundance of immune-related proteins (Diaz et al., 2016), and packaging bacterial molecules within host-derived exosomes (Giri et al., 2010; Kruh-Garcia et al., 2014). M. tuberculosis proteins within these exosomes activate the host immune response in the recipient cell (Giri et al., 2010) (Fig. 2A). Interestingly, exosome release increases during M. tuberculosis infection of bone marrow-derived macrophages (BMDMs) and wild-type or Rab27a−/− mice (Smith et al., 2017). The importance of exosome secretion during M. tuberculosis infection was determined using Rab27a−/− mice, as Rab27 is necessary for docking and tethering of MVBs (Ostrowski et al., 2010). Infection of Rab27a−/− mice with M. tuberculosis results in higher bacterial burden and diminished CD4+ T cell activation than for wild-type mice (Smith et al., 2017). Furthermore, treatment of bone marrow-derived macrophages with exosomes purified from infected Rab27a−/− mice leads to diminished secretion of chemokine ligand 1 (CCL1), interleukin 1a (IL-1a), chemokine ligand 2 (CXCL2), CCL5 and tumor necrosis factor (TNF) when compared to the control treatment. The reduced secretion of these pro-inflammatory factors results in inefficient immune activation and bacterial clearance (Smith et al., 2017). Taken together, these findings indicate that exosome biogenesis, and specifically Rab27a, is necessary for an immune response against M. tuberculosis.
Fig. 2.
Extracellular vesicles and infection. (A) Immune activation in the mouse model. M. tuberculosis modifies the protein content of host extracellular vesicles (EVs) during intracellular infection. Secretion of modified EVs is dependent on Rab27a. Interaction of modified EVs with uninfected macrophages and activated T cells leads to the secretion of chemokines and cytokines, thus, eliciting an immune response. (B) Immune evasion in the mouse model. Parasitic nematodes manipulate host immune responses in the intestine by secreting exosomes. These exosomes contain regulatory small RNAs, which decrease pro-inflammatory responses, activation of alternatively activated macrophages (AAMФ) and eosinophil migration. Binding of these nematode vesicles to mammalian cells suppresses the expression of Il1rl1, Mpk1 and the mannose receptor CD206, as well as the secretion of resistin-like molecule α (RELMα), Ym1 and several cytokines. (C) Invasion of hepatic cells. Hepatitis virus A (HAV) hijacks the exosome biogenesis accessory proteins ALIX and VSP4 to form an envelope (eHAV) that protects the virus from neutralizing antibodies. (D) Pathogenesis of amoeba to mammalian cells. The pathogenic free-living amoeba Acanthamoeba castellanii secretes EVs that contain metalloproteases and serine proteases that are taken up in the model CHO and T98G cell lines. Once EVs are endocytosed, the cargo leads to apoptosis of host cells, possibly, causing tissue damage that is associated with Acanthamoeba keratitis.
Immune evasion
Several parasites use extracellular vesicles to diminish host immune responses (Buck et al., 2014; Eichenberger et al., 2018; Singh et al., 2011). Heligmosomoides polygyrus, a gastrointestinal nematode, secretes exosomes that are structurally distinct in their lipid signature when compared to host exosomes (Simbari et al., 2016). These parasitic exosomes contain miRNAs, which are similar to host small RNAs. Additionally, they carry Y RNAs and argonaute, a protein required for RNA-mediated gene silencing. Treatment of mammalian cells with H. polygyrus-derived exosomes results in downregulation of mitogen-activated protein kinase 1 (Mkp1) and interleukin 1 receptor-like 1 (Il1rl1) gene transcription (Buck et al., 2014). H. polygyrus-derived exosomes also mitigate eosinophil migration through the reduced secretion of IL-5 and IL-13 (Buck et al., 2014) (Fig. 2B). Interestingly, H. polygyrus-derived vesicles are preferentially internalized by macrophages activated through the alternative pathway (M2; AAMФ), which are important during anti-parasite immune responses. Treatment of pre-AAMФ and AAMФ macrophages with these vesicles results in decreased secretion of resistin-like molecule α (RELMα), Ym1 (also known as CHIL3), IL-10 and the expression of the mannose receptor CD206 (also known as MRC1) and Il1rl1 (Coakley et al., 2017). These findings demonstrate that nematode vesicles reduce anti-parasite immune responses. In addition, extracellular vesicles from another parasitic nematode, Nippostrongylus brasiliensis, mitigate host defenses (Eichenberger et al., 2018). Thus, an emerging concept in the field is that extracellular vesicles may have anti-inflammatory features.
Viral invasion
A recent study described a role for vesicles coated with the ALG-2-interacting protein X (ALIX; also known as PDCD6IP), which is associated with the ESCRT-III complex, in the packaging of the non-enveloped hepatitis A virus (HAV). This vesicle-bound form of HAV is referred to as ‘enveloped hepatitis A virus’ (eHAV) (Fig. 2C); eHAVs are infectious, contain a capsid antigen and are resistant to neutralizing antibodies (Feng et al., 2013). Interestingly, reducing the expression of VSP4 and ALIX affects the release of eHAVs, but not other ESCRT components (Feng et al., 2013). Moreover, the human immunodeficiency virus (HIV) and herpes simplex virus 1 (HSV-1) exploit extracellular vesicles to enhance their spread into uninfected cells (Arakelyan et al., 2017). Conceptually, an emerging paradigm is that viruses may hijack the extracellular vesicle machinery to invade mammalian cells.
Pathogenesis
Acanthamoeba castellanii is a free-living amoeba that causes the eye infection Acanthamoeba keratitis. The disease pathology is primarily associated with metalloproteases and serine proteases at the site of infection, which are secreted by A. castellanii within nanovesicles and absorbed by mammalian cells to promote apoptosis (Gonçalves et al., 2018) (Fig. 2D). Although the exact mechanism that leads to cell apoptosis is unknown, it was determined that the toxicity of vesicles to mammals was dependent on the activity of the proteases within their cargo. Cell death at the infectious site by A. castellanii through extracellular vesicles is likely connected with the pathology of ocular keratitis (Gonçalves et al., 2018). Other examples of how vesicles promote pathogenesis during infectious diseases are discussed below.
Extracellular vesicles and arthropod-borne microbial transmission
The discipline of parasitology is at the forefront of research for extracellular vesicles and vector-borne diseases. Once inside the host or the arthropod, some parasites appear to hijack the extracellular vesicle machinery to tailor the mammalian immune response. Here, we will elaborate on how some parasites manipulate the host machinery, before discussing how cell-to-cell communication between the arthropod vector, the mammalian host and the microbial pathogen affects molecular signaling in order to promote or hinder infection.
Molecular interactions between parasites and the mammalian host
Plasmodium spp.
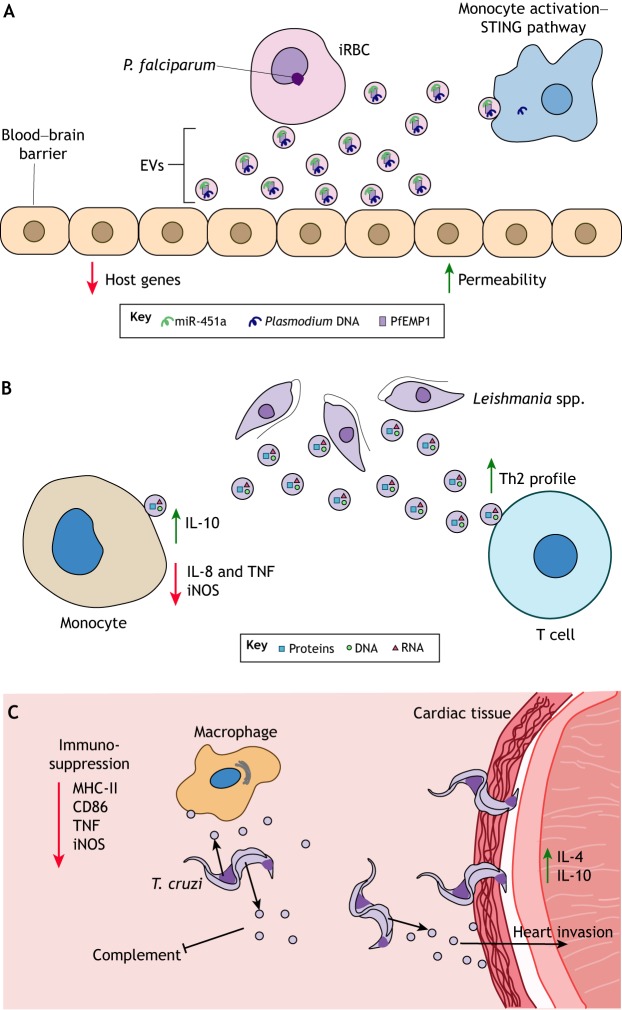
Malaria is a deadly vector-borne illness caused by Plasmodium spp. These parasites are deposited into the skin by mosquitoes and eventually spread to the liver. Following an incubation period in the liver, parasites are released into the bloodstream and infect red blood cells. Much of the research surrounding malaria extracellular vesicles has been focused at the blood stage, specifically on characterizing vesicles secreted by infected red blood cells (iRBCs) (Fig. 3A). Subsequent to infection with malaria parasites, iRBCs secrete significantly more extracellular vesicles than uninfected cells (Mantel et al., 2016; Regev-Rudzki et al., 2013). Extracellular vesicles derived from iRBCs contain many factors, including antigens, virulence factors, mammalian- and parasite-derived nucleic acids, and the RNAi machinery (Babatunde et al., 2018; Mantel et al., 2016; Sampaio et al., 2018; Wang et al., 2017).
Fig. 3.
Extracellular vesicles affect host responses during vector-borne pathogen infection. (A) Cerebral malaria in humans is linked to damage of the blood–brain barrier. These symptoms may be associated with extracellular vesicle (EV) secretion by P. falciparum-infected red blood cells (iRBCs). These vesicles contain the P. falciparum erythrocyte membrane protein 1 (PfEMP1), host miR-451a and DNA. PfEMP1 and miR-451a increase the permeability of the cell barrier through the downregulation of host gene expression. In addition, P. falciparum DNA in these vesicles activate immune responses in circulating monocytes through the stimulator of interferon genes (STING) pathway. (B) Leishmania spp., the causative agent of Leishmaniasis, secrete EVs during host infection. As shown in mice, these vesicles are taken up by monocytes and T cells, where they suppress the secretion of pro-inflammatory cytokines and steer T cell differentiation towards Th2 responses. (C) The Chagas disease parasite Trypanosoma cruzi secretes EVs that exert an immunosuppressive activity in the human host by blocking complement formation, suppressing macrophage antigen presentation (MHC-II), inhibiting T cell stimulation through CD86, dampening the secretion of TNF and the expression of iNOS, and increasing invasion of cardiac tissue through the elevated levels of IL-4 and IL-10, leading to tissue damage in the heart.
Extracellular vesicles from Plasmodium iRBCs contribute to the severity of disease, specifically to vascular dysfunction during cerebral malaria (El-Assaad et al., 2014). Recently, it has been reported that extracellular vesicles from iRBCs contain complexes of miRNAs and argonaute-2 (Mantel et al., 2016). Delivery of vesicle cargo miRNAs, specifically human miR-451a, contributes to the downregulation of genes involved in endothelial barrier function (Fig. 3A). Indeed, miRNA delivery by vesicles has been shown to increase permeability of the endothelium, indicating a potential means for parasites to breach the blood–brain barrier (Mantel et al., 2016). Moreover, P. falciparum erythrocyte membrane protein 1 (PfEMP1), which is involved in iRBC adherence and sequestration in blood vessels, was identified in extracellular vesicles (Sampaio et al., 2018). Regulation of host gene expression and increased endothelial adherence suggests a mechanism of enhanced disease severity that is mediated by extracellular vesicles.
iRBCs secrete vesicles that contain parasite nucleic acids (Babatunde et al., 2018; Sisquella et al., 2017), including Plasmodium genomic DNA, which may activate cytosolic DNA sensors in monocytes (Sisquella et al., 2017). Delivery of parasite DNA also promoted type I interferon and chemokine production, which is indicative of a stimulator of interferon genes (STING) response (Fig. 3A) (Sisquella et al., 2017). The transport of parasitic cargo between cells demonstrates the important role that vesicles play in engaging host immune responses.
Leishmania spp.
Leishmania spp. are a group of over twenty protozoan parasites that are transmitted by various species of sandfly. Leishmaniasis manifests into three clinical forms: cutaneous, mucocutaneous and visceral leishmaniasis. Leishmania spp. were first shown to secrete exosomes by Silverman and colleagues, who observed that extracellular vesicle release increases at the mammalian host temperature (37°C) and specific cargo is enriched at acidic pH, representing conditions during an early infection (Silverman et al., 2010a). Unlike what is seen with other intracellular pathogens, infection with Leishmania parasites alone does not increase extracellular vesicle secretion (Cronemberger-Andrade et al., 2014). Leishmania promastigote exosomes carry heat-shock proteins and virulence factors, including the metalloprotease GP63 and the protein tyrosine phosphatase L. major (Lm)-PRL-1, which contribute to parasite survival in the mammalian host (Leitherer et al., 2017; Silverman et al., 2010a). Leishmania exosomes isolated from amastigotes contain siRNAs and tRNA-derived small RNAs (Lambertz et al., 2015). The RNA content of Leishmania spp. has a surprisingly high degree of similarity between the types found in the Old and New World despite them being transmitted by different vectors. Collectively, these results therefore suggest a conserved mechanism for parasite RNA packaging.
Exosomes derived from Leishmania parasites and infected macrophages aid in parasite survival. Indeed, it was demonstrated that pretreating monocytes with exosomes derived from L. donovani upregulates the production of IL-10 and decreases that of IL-8 and TNF, suggesting that they have an immunosuppressive role (Silverman et al., 2010b) (Fig. 3B). L. major exosomes appear to promote T helper 2 (Th2) cell polarization, which is an adaptive immune response permissive to Leishmania infection (Silverman et al., 2010b). Specifically, L. major exosomes downregulate the expression of inducible nitric oxide synthase (iNOS, also known as NOS3) and promote activation of protein tyrosine phosphatases (PTPs), resulting in impaired parasite killing by macrophages. This immunomodulation was shown to be dependent on the expression of Leishmania GP63 (Hassani et al., 2014). Taken together, Leishmania exosomes drive an immune response that favors parasite infection by limiting inflammation.
Trypanosoma spp.
Trypanosomes are unicellular flagellate protozoa divided into two major classes: salivarian and stercorarian (Kaufer et al., 2017). T. cruzi is a stercorarian trypanosome that is transmitted by triatomine bugs and causes Chagas disease. Even by 1991, the shedding of surface antigens by membrane vesicles from T. cruzi had been observed (Gonçalves et al., 1991). Trypomastigote vesicles carry molecules involved in nucleic acid binding, heat-shock proteins and mucins, as well as parasite surface components (Bayer-Santos et al., 2013; Gonçalves et al., 1991; Nogueira et al., 2015). Because T. cruzi comprises several groups and subgroups, there are strain-dependent differences in vesicle protein content, pathogenesis and immune evasion (Neves et al., 2014; Ribeiro et al., 2018; Wyllie and Ramirez, 2017).
Extracellular vesicles from Trypanosoma spp. possess immunomodulatory properties that contribute to parasite survival. Once T. cruzi enters the host through a wound and penetrates the mucous membranes, it arrives in the bloodstream and becomes a target of the complement system. As a mechanism of host immune evasion, both T. cruzi trypomastigotes and the host blood cells that interact with them secrete vesicles that promote resistance to the lectin pathway of complement-mediated lysis (Wyllie and Ramirez, 2017) (Fig. 3C). Although the exact mechanism is unknown, it was speculated that this could be due to the inhibition of the C3 convertase, which is a key component during the complement activation (Lidani et al., 2017; Wyllie and Ramirez, 2017). Interestingly, vesicles from one strain do not confer complement resistance to trypanosomes from a different group (Wyllie and Ramirez, 2017). This strain-dependent resistance highlights the level of specificity in the ability of the parasite to protect itself from the immune system of its host.
Extracellular vesicles derived from T. cruzi also promote an immunosuppressive response through the induction of IL-4 and IL-10 in the heart tissue, and a reduction of iNOS and TNF production by macrophages (Lovo-Martins et al., 2018; Trocoli Torrecilhas et al., 2009). Accordingly, priming of macrophages with extracellular vesicles from T. cruzi results in fewer macrophages that are positive for MHC class II molecules, suggesting that vesicles can suppress macrophage stimulation and dampen T cell activation (Lovo-Martins et al., 2018). Such an immunosuppression is further indicated by the decreased expression of CD86 (B7-2), a co-stimulatory molecule involved in T cell activation (Lovo-Martins et al., 2018).
T. cruzi-derived vesicles also contain phosphatases that increase parasite adhesion to host macrophages (Neves et al., 2014). Pretreatment with vesicles from T. cruzi has been shown to contribute to increased invasion of heart tissues by the parasite, with exacerbated lesions and higher mortality (Trocoli Torrecilhas et al., 2009). As Chagas disease is primarily a disease of the heart and the nervous system, this enhanced pathology emphasizes the important role of extracellular vesicles in promoting disease.
Another example of how nanovesicles are involved in the pathogenesis of a trypanosome is the case of T. brucei, a salivarian trypanosome that causes African sleeping sickness (African trypanosomiasis). These parasites produce filamentous structures termed ‘membrane nanotubes’ that release extracellular vesicles (Szempruch et al., 2016). These vesicles rapidly fuse with the membranes of mammalian red blood cells and alter their composition, promoting erythrophagocytosis and resulting in a smaller number of circulating red blood cells (Szempruch et al., 2016). Decreased number of red blood cells provokes the development of anemia, which is the primary cause of morbidity associated with African trypanosomiasis. These results indicate the importance of T. brucei nanovesicles in mediating disease pathology.
Brugia spp.
Brugia malayi, the causative agent of lymphatic filariasis, is a parasitic roundworm spread by mosquitoes. During a blood meal, mosquitoes deposit larvae into the skin, which then develop as adult worms within the lymphatics. Brugia malayi eventually reaches the bloodstream as microfilariae. Extracellular vesicles from Brugia spp. are internalized by macrophages (Zamanian et al., 2015) and secreted during all intra-mammalian life cycle stages (Harischandra et al., 2018). The protein content of these vesicles differs depending on the life cycle stage and the sex of the worms (Harischandra et al., 2018). For instance, vesicles secreted by female Brugia malayi are enriched in immunomodulatory molecules, such as bioactive microRNAs (Zamanian et al., 2015). Interestingly, RNAseq analysis has identified miRNAs in Brugia spp. vesicles that are homologous to host miRNAs, including miRNAs that are either identical or similar to let-7. The let-7 miRNA family targets genes involved in innate immune pathways, cell proliferation and macrophage polarization (Banerjee et al., 2013; Teng et al., 2013), suggesting a possible mechanism for how this extracellular parasite modulates the host immune response.
Molecular interactions between arthropod vectors and parasites
Mosquitoes, kissing bugs and sandflies spread many pathogens. Therefore, the role of extracellular vesicles in the transmission of pathogens through their vectors has become an attractive area of research. In this section, we will discuss some of these guiding principles.
Leishmania spp.
During the Leishmania life cycle, parasites infect the midgut of sandflies before being transmitted to a mammalian host. Leishmania parasites constitutively release exosome-like vesicles directly into the sandfly midgut (Atayde et al., 2015) (Fig. 4A). These vesicles are found in the sandfly inoculum and are subsequently co-egested with parasites during the blood meal. Extracellular vesicles derived from midgut-isolated Leishmania also carry virulence and immunological factors, including GP63 and Hsp70 (Atayde et al., 2015). Importantly, these extracellular vesicles appear to worsen the disease pathology. Co-inoculation of L. major parasites with purified exosomes increased the lesion size and footpad swelling in mice. Mice injected with both exosomes and Leishmania also had higher parasite loads in non-necrotic lesions and increased secretion of a number of cytokines, including IL-2, IL-4, IFN-γ, IL-17a, IL-23 and IL-10, than those injected with Leishmania alone (Atayde et al., 2015). Hence, these findings demonstrate that extracellular vesicles are relevant in the arthropod vector and clearly influence the mammalian immune response.
Fig. 4.
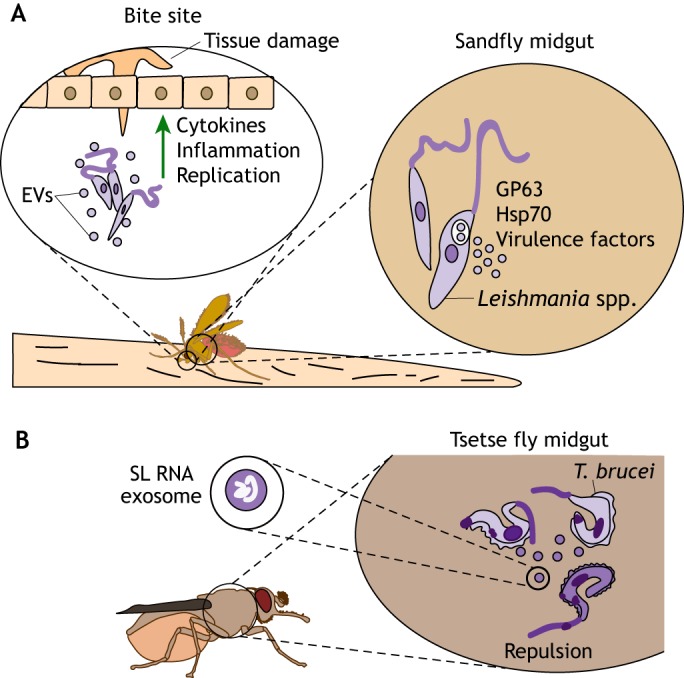
Parasite-derived extracellular vesicles influence vector–host interactions. (A) During replication within the sandfly midgut, Leishmania spp. secrete extracellular vesicles (EVs) that contain virulence factors and heat-shock proteins, such as GP63 and Hsp7. Blood-feeding sandflies inject these EVs into the bite site, along with the parasites, thereby stimulating the secretion of pro-inflammatory cytokines, increasing tissue damage and enhancing pathogen replication, which aggravates the disease. (B) EVs are involved in environmental sensing and signaling during replication within arthropod vectors. T. brucei uses EVs to signal stress conditions to other replicating parasites inside the midgut of tsetse flies. Unfit parasites (dark purple) secrete EVs containing SL RNA, which accumulates when mRNA trans-splicing is disrupted. These EVs are taken up by healthy parasites (light purple), leading to an arrest in their movement and/or change in direction. Intercommunication through EVs may enable successful vector colonization by these parasites to avoid an environment that is unfit for replication.
Trypanosoma spp.
Extracellular vesicles secreted within tsetse flies have an important role in the communication between trypanosomes, particularly during social motility (Eliaz et al., 2017). T. brucei move through semi-solid surfaces in swarms. ‘Scouts’ sense the environmental conditions at the edges and send signals to other individuals within the swarm to guide their movement, in a process defined as ‘social motility’ (Oberholzer et al., 2010). Furthermore, it has been postulated that trypanosomes send stress-condition signals into the environment to halt parasite movement (Eliaz et al., 2017). In experiments with T. brucei, it has been demonstrated that the procyclic forms typically found in the midgut of the tsetse fly are capable of secreting extracellular vesicles when trans-splicing of mRNAs is disrupted (Eliaz et al., 2017). During disrupted trans-splicing, the spliced leader (SL) RNA begins accumulating in the parasite, which leads to the formation of granules (Fig. 4B). These SL RNA-containing granules are secreted by the affected trypanosome in the form of exosomes and are then endocytosed by neighboring parasites. Intriguingly, secreted exosomes significantly enhance the repulsion observed between parasites. Repulsion signals from unfit parasites could be a method of promoting a successful colonization of the tsetse fly (Eliaz et al., 2017), suggesting another mechanism of vesicle-mediated communication to ensure an infection.
Extracellular vesicles secreted by arthropod vectors and viral transmission
Extracellular vesicles originating from arthropod vectors are becoming apparent as an important strategy for immune evasion during microbial transmission. During infection with Langat virus (LGTV), the tick cell line ISE6 (Oliver et al., 2015) secretes exosomes that contain cargo from both the virus and the vector (Zhou et al., 2018). Indeed, tick cells infected with LGTV release higher numbers of exosomes than uninfected cells, and these exosomes can transmit the virus to an uninfected organism. Interestingly, virus purified from infected exosomes are also capable of colonizing human HaCaT keratinocytes (Zhou et al., 2018). These findings reveal an intriguing interaction between vector-derived vesicles and mammalian host cells. Similarly, other viruses hijack extracellular vesicles from the arthropod vector for their transmission. For instance, the Dengue virus serotype 2 (DENV2) uses extracellular vesicles derived from mosquitoes to infect mammalian cells (Vora et al., 2018). During Dengue virus infection, mosquito-derived vesicles carry viral proteins and a full-length DENV2 genome. Importantly, DENV2 transmission is dependent on vesicle secretion by the vector, which may occur through the interaction between the tetraspanin domain-containing glycoprotein Tsp29Fb, a mosquito homolog of the exosomal marker CD63, and the viral E protein (Vora et al., 2018). Although these findings were primarily obtained in vitro, they clearly suggest that arthropod extracellular vesicles may transmit viruses. Future efforts are needed to fully elucidate the contribution of vector-derived extracellular vesicles in pathogen transmission at the vector–host interface.
Perspectives
Within the past decade, research on extracellular vesicles has increased exponentially in the field of infectious diseases. This is partially due to the better-appreciated role these vesicles have in directing host immunity. Although we do not fully understand how arthropod vectors aid in the establishment of illnesses, an emerging principle is that pathogens exploit extracellular vesicles secreted by their vectors. Indeed, independent groups have shown that vector-borne microbes can change the protein and miRNA content of vector and host vesicles by adding pathogen-derived components that assist in microbial spreading (Sampaio et al., 2018; Vora et al., 2018; Zhou et al., 2018). Dissection of these underlying mechanisms is clearly a priority, and there are outstanding questions that should be explored. For instance, do microbial pathogens inhibit inflammation by modifying the oxidative state of arthropod vesicles? Do the skin microbiome and metabolites present at the bite site influence the content of arthropod vesicles? Furthermore, what is the contribution of different molecular entities (e.g. nucleic acids, carbohydrates, lipids or proteins) to an immune response against a microbial pathogen and/or an arthropod vector?
Blood-feeding arthropods secrete a vast arsenal of immunomodulatory molecules (Leitner et al., 2013; Shaw et al., 2016; Šimo et al., 2017). How these effector molecules affect immune signaling is another active area of investigation. Experimental evidence suggests that SNAREs are important for vector feeding (Browning and Karim, 2013; Karim et al., 2005; Villarreal et al., 2013), indicating that these effectors may be secreted inside nanovesicles. Saliva from different arthropod species carry classical markers of extracellular vesicles (Díaz-Martín et al., 2013; Tirloni et al., 2015, 2014), as well as anti-inflammatory miRNAs (Hackenberg and Kotsyfakis, 2018). These observations suggest that nanovesicles may modulate the host immune response at the bite site, resulting in successful feeding. How does vesicle secretion assist arthropod vectors during feeding? Does arthropod feeding show a different dynamic state when abiotic factors (e.g. temperature) and biotic factors (e.g. commensals) are present? Moreover, how does prolonged versus short arthropod feeding affect trans-kingdom intercommunication? Are secretory mechanisms of extracellular vesicles conserved between vertebrate and non-vertebrate animals?
If the underlying mechanics of vesicle secretion are evolutionarily conserved among blood-feeding arthropods, it becomes paramount to identify cellular targets. Equally important will be to define the transduction networks that are affected by vesicles during pathogen transmission. For example, it remains unclear whether vector-secreted vesicles interact with mammalian cells through endocytosis or receptor-mediated processes. Similarly, we do not yet know whether ligands present on the vesicle membranes dictate the organotropism of arthropod vesicles. For example, integrins direct the organotropism of cancer cell-derived exosomes (Hoshino et al., 2015). Thus, it is possible that integrins on the membranes of vector-derived vesicles may govern the interaction of exosomes with specific cells and organs. In this regard, another interesting question is whether the effect of arthropod vesicles is limited to the regulation of immune responses, or whether they also affect metabolism and physiology? Does the extent of co-evolutionary history between vectors and pathogens affect vesicle secretion?
In this Review, we attempted to summarize the current knowledge of extracellular vesicles in vector-borne diseases. We also highlighted the most critical questions that would fundamentally advance the field of arthropod-borne illnesses. Such an undertaking is monumental because genetic manipulation is unavailable for most arthropod vectors. Similarly, specific tools that completely abolish vesicle secretion in vivo have not been developed. However, with the advent of genome editing and the power of systems biology, mechanistic analysis focusing on the arthropod vector is becoming more realistic.
Acknowledgements
We would like to thank Erin McClure Carroll for editing this manuscript. We also acknowledge Holly Hammond for assistance with the illustrations.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Our work in this area is supported by grants from the National Institutes of Health (NIH) to J.H.F.P. (P01AI138949, R01AI116523, R01AI134696 and subcontract recipient for R01AI049424). Deposited in PMC for release after 12 months.
References
- Altan-Bonnet N. (2016). Extracellular vesicles are the Trojan horses of viral infection. Curr. Opin. Microbiol. 32, 77-81. 10.1016/j.mib.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakelyan A., Fitzgerald W., Zicari S., Vanpouille C. and Margolis L. (2017). Extracellular vesicles carry HIV Env and facilitate HIV infection of human lymphoid tissue. Sci. Rep. 7, 1695 10.1038/s41598-017-01739-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atayde V. D., Aslan H., Townsend S., Hassani K., Kamhawi S. and Olivier M. (2015). Exosome secretion by the parasitic protozoan Leishmania within the sand fly midgut. Cell Rep. 13, 957-967. 10.1016/j.celrep.2015.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babatunde K. A., Mbagwu S., Hernández-Castañeda M. A., Adapa S. R., Walch M., Filgueira L., Falquet L., Jiang R. H. Y., Ghiran I. and Mantel P.-Y. (2018). Malaria infected red blood cells release small regulatory RNAs through extracellular vesicles. Sci. Rep. 8, 884 10.1038/s41598-018-19149-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baietti M. F., Zhang Z., Mortier E., Melchior A., Degeest G., Geeraerts A., Ivarsson Y., Depoortere F., Coomans C., Vermeiren E. et al. (2012). Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 14, 677-685. 10.1038/ncb2502 [DOI] [PubMed] [Google Scholar]
- Banerjee S., Xie N., Cui H., Tan Z., Yang S., Icyuz M., Abraham E. and Liu G. (2013). MicroRNA let-7c regulates macrophage polarization. J. Immunol. 190, 6542-6549. 10.4049/jimmunol.1202496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer-Santos E., Aguilar-Bonavides C., Rodrigues S. P., Cordero E. M., Marques A. F., Varela-Ramirez A., Choi H., Yoshida N., da Silveira J. F. and Almeida I. C. (2013). Proteomic analysis of Trypanosoma cruzi secretome: characterization of two populations of extracellular vesicles and soluble proteins. J. Proteome Res. 12, 883-897. 10.1021/pr300947g [DOI] [PubMed] [Google Scholar]
- Beer K. B., Rivas-Castillo J., Kuhn K., Fazeli G., Karmann B., Nance J. F., Stigloher C. and Wehman A. M. (2018). Extracellular vesicle budding is inhibited by redundant regulators of TAT-5 flippase localization and phospholipid asymmetry. Proc. Natl. Acad. Sci. USA 115, E1127-E1136. 10.1073/pnas.1714085115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning R. and Karim S. (2013). RNA interference-mediated depletion of N-ethylmaleimide sensitive fusion protein and synaptosomal associated protein of 25 kDa results in the inhibition of blood feeding of the Gulf Coast tick, Amblyomma maculatum. Insect Mol. Biol. 22, 245-257. 10.1111/imb.12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck A. H., Coakley G., Simbari F., McSorley H. J., Quintana J. F., Le Bihan T., Kumar S., Abreu-Goodger C., Lear M., Harcus Y. et al. (2014). Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat. Commun. 5, 5488 10.1038/ncomms6488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolukbasi M. F., Mizrak A., Ozdener G. B., Madlener S., Ströbel T., Erkan E. P., Fan J. B., Breakefield X. O. and Saydam O. (2012) miR-1289 and “Zipcode”-like sequence enrich mRNAs in microvesicles. Mol. Ther. Nucleic Acids1, e10 10.1038/mtna.2011.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshomi H. and Matin M. M. (2018). Exosomes and their importance in metastasis, diagnosis, and therapy of colorectal cancer. J. Cell. Biochem. 23, 27582 10.1002/jcb.27582 [DOI] [PubMed] [Google Scholar]
- Choi H. W., Suwanpradid J., Kim I. H., Staats H. F., Haniffa M., MacLeod A. S. and Abraham S. N. (2018). Perivascular dendritic cells elicit anaphylaxis by relaying allergens to mast cells via microvesicles. Science 362, eaao0666 10.1126/science.aao0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy J. W., Sedgwick A., Rosse C., Muralidharan-Chari V., Raposo G., Method M., Chavrier P. and D'Souza-Schorey C. (2015). Regulated delivery of molecular cargo to invasive tumour-derived microvesicles. Nat. Commun. 6, 6919 10.1038/ncomms7919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coakley G., McCaskill J. L., Borger J. G., Simbari F., Robertson E., Millar M., Harcus Y., McSorley H. J., Maizels R. M. and Buck A. H. (2017). Extracellular vesicles from a helminth parasite suppress macrophage activation and constitute an effective vaccine for protective immunity. Cell Rep. 19, 1545-1557. 10.1016/j.celrep.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M., Moita C., van Niel G., Kowal J., Vigneron J., Benaroch P., Manel N., Moita L. F., Théry C. and Raposo G. (2013). Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 126, 5553-5565. 10.1242/jcs.128868 [DOI] [PubMed] [Google Scholar]
- Cronemberger-Andrade A., Aragão-França L., de Araujo C. F., Rocha V. J., Borges-Silva M. C., Figueira C. P., Oliveira P. R., de Freitas L. A. R., Veras P. S. T. and Pontes-de-Carvalho L. (2014). Extracellular vesicles from Leishmania-infected macrophages confer an anti-infection cytokine-production profile to naïve macrophages. PLoS Negl. Trop. Dis. 8, e3161 10.1371/journal.pntd.0003161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz G., Wolfe L. M., Kruh-Garcia N. A. and Dobos K. M. (2016). Changes in the membrane-associated proteins of exosomes released from human macrophages after Mycobacterium tuberculosis infection. Sci. Rep. 6, 37975 10.1038/srep37975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Martín V., Manzano-Román R., Valero L., Oleaga A., Encinas-Grandes A. and Pérez-Sánchez R. (2013). An insight into the proteome of the saliva of the argasid tick Ornithodoros moubata reveals important differences in saliva protein composition between the sexes. J. Proteomics 80, 216-235. 10.1016/j.jprot.2013.01.015 [DOI] [PubMed] [Google Scholar]
- Eichenberger R. M., Ryan S., Jones L., Buitrago G., Polster R., Montes de Oca M., Zuvelek J., Giacomin P. R., Dent L. A., Engwerda C. R. et al. (2018). Hookworm secreted extracellular vesicles interact with host cells and prevent inducible colitis in mice. Front. Immunol. 9, 850 10.3389/fimmu.2018.00850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Assaad F., Wheway J., Hunt N. H., Grau G. E. R. and Combes V. (2014). Production, fate and pathogenicity of plasma microparticles in murine cerebral malaria. PLoS Pathog. 10, e1003839 10.1371/journal.ppat.1003839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliaz D., Kannan S., Shaked H., Arvatz G., Tkacz I. D., Binder L., Waldman Ben-Asher H., Okalang U., Chikne V., Cohen-Chalamish S. et al. (2017). Exosome secretion affects social motility in Trypanosoma brucei. PLoS Pathog. 13, e1006245 10.1371/journal.ppat.1006245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader C. M., Sánchez D. G., Mestre M. B. and Colombo M. I. (2009). TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim. Biophys. Acta 1793, 1901-1916. 10.1016/j.bbamcr.2009.09.011 [DOI] [PubMed] [Google Scholar]
- Feng Z., Hensley L., McKnight K. L., Hu F., Madden V., Ping L. F., Jeong S.-H., Walker C., Lanford R. E. and Lemon S. M. (2013). A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 496, 367-371. 10.1038/nature12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A., Sampey G., Chung M.-C., Bailey C., van Hoek M. L., Kashanchi F. and Hakami R. M. (2014). The carrying pigeons of the cell: exosomes and their role in infectious diseases caused by human pathogens. Pathog. Dis. 71, 109-120. 10.1111/2049-632X.12135 [DOI] [PubMed] [Google Scholar]
- Giri P. K., Kruh N. A., Dobos K. M. and Schorey J. S. (2010). Proteomic analysis identifies highly antigenic proteins in exosomes from M. tuberculosis-infected and culture filtrate protein-treated macrophages. Proteomics 10, 3190-3202. 10.1002/pmic.200900840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves M. F., Umezawa E. S., Katzin A. M., de Souza W., Alves M. J., Zingales B. and Colli W. (1991). Trypanosoma cruzi: shedding of surface antigens as membrane vesicles. Exp. Parasitol. 72, 43-53. 10.1016/0014-4894(91)90119-H [DOI] [PubMed] [Google Scholar]
- Gonçalves D. S., Ferreira M. S., Liedke S. C., Gomes K. X., de Oliveira G. A., Leão P. E. L., Cesar G. V., Seabra S. H., Cortines J. R., Casadevall A. et al. (2018). Extracellular vesicles and vesicle-free secretome of the protozoa Acanthamoeba castellanii under homeostasis and nutritional stress and their damaging potential to host cells. Virulence 9, 818-836. 10.1080/21505594.2018.1451184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. C., Chaudhary V., Bartscherer K. and Boutros M. (2012). Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 14, 1036-1045. 10.1038/ncb2574 [DOI] [PubMed] [Google Scholar]
- Hackenberg M. and Kotsyfakis M. (2018). Exosome-mediated pathogen transmission by arthropod vectors. Trends Parasitol. 34, 549-552. 10.1016/j.pt.2018.04.001 [DOI] [PubMed] [Google Scholar]
- Harischandra H., Yuan W., Loghry H. J., Zamanian M. and Kimber M. J. (2018). Profiling extracellular vesicle release by the filarial nematode Brugia malayi reveals sex-specific differences in cargo and a sensitivity to ivermectin. PLoS Negl. Trop. Dis. 12, e0006438 10.1371/journal.pntd.0006438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani K., Shio M. T., Martel C., Faubert D. and Olivier M. (2014). Absence of metalloprotease GP63 alters the protein content of Leishmania exosomes. PLoS ONE 9, e95007 10.1371/journal.pone.0095007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A., Costa-Silva B., Shen T.-L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S. et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527, 329-335. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S., Miller N. J., Valenzuela J., Sauer J. R. and Mather T. N. (2005). RNAi-mediated gene silencing to assess the role of synaptobrevin and cystatin in tick blood feeding. Biochem. Biophys. Res. Commun. 334, 1336-1342. 10.1016/j.bbrc.2005.07.036 [DOI] [PubMed] [Google Scholar]
- Kaufer A., Ellis J., Stark D. and Barratt J. (2017). The evolution of trypanosomatid taxonomy. Parasit. Vectors 10, 287 10.1186/s13071-017-2204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruh-Garcia N. A., Wolfe L. M., Chaisson L. H., Worodria W. O., Nahid P., Schorey J. S., Davis J. L. and Dobos K. M. (2014). Detection of Mycobacterium tuberculosis peptides in the exosomes of patients with active and latent M. tuberculosis infection using MRM-MS. PLoS ONE 9, e103811 10.1371/journal.pone.0103811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertz U., Oviedo Ovando M. E., Vasconcelos E. J., Unrau P. J., Myler P. J. and Reiner N. E. (2015). Small RNAs derived from tRNAs and rRNAs are highly enriched in exosomes from both old and new world Leishmania providing evidence for conserved exosomal RNA packaging. BMC Genomics 16, 151 10.1186/s12864-015-1260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitherer S., Clos J., Liebler-Tenorio E. M., Schleicher U., Bogdan C. and Soulat D. (2017). Characterization of the protein tyrosine phosphatase LmPRL-1 secreted by Leishmania major via the exosome pathway. Infect. Immun. 85, 00084-17 10.1128/IAI.00084-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner W. W., Wali T. and Costero-Saint Denis A. (2013). Is arthropod saliva the achilles’ heel of vector-borne diseases? Front. Immunol. 4, 255 10.3389/fimmu.2013.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Antonyak M. A., Zhang J. and Cerione R. A. (2012). RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31, 4740-4749. 10.1038/onc.2011.636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidani K. C. F., Bavia L., Ambrosio A. R. and de Messias-Reason I. J. (2017). The complement system: a prey of Trypanosoma cruzi. Front. Microbiol. 8, 607 10.3389/fmicb.2017.00607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovo-Martins M. I., Malvezi A. D., Zanluqui N. G., Lucchetti B. F. C., Tatakihara V. L. H., Mörking P. A., de Oliveira A. G., Goldenberg S., Wowk P. F. and Pinge-Filho P. (2018). Extracellular vesicles shed by Trypanosoma cruzi potentiate infection and elicit lipid body formation and PGE2 production in murine macrophages. Front. Immunol. 9, 896 10.3389/fimmu.2018.00896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunavat T. R., Cheng L., Kim D.-K., Bhadury J., Jang S. C., Lässer C., Sharples R. A., López M. D., Nilsson J., Gho Y. S. et al. (2015). Small RNA deep sequencing discriminates subsets of extracellular vesicles released by melanoma cells--evidence of unique microRNA cargos. RNA Biol. 12, 810-823. 10.1080/15476286.2015.1056975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel P.-Y., Hjelmqvist D., Walch M., Kharoubi-Hess S., Nilsson S., Ravel D., Ribeiro M., Grüring C., Ma S., Padmanabhan P. et al. (2016). Infected erythrocyte-derived extracellular vesicles alter vascular function via regulatory Ago2-miRNA complexes in malaria. Nat. Commun. 7, 12727 10.1038/ncomms12727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M. and Johnson P. J. (2016). Emerging roles for extracellular vesicles in parasitic infections. Curr. Opin. Microbiol. 32, 66-70. 10.1016/j.mib.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy E. M., Wilkinson F. L., Parker B. and Alexander M. Y. (2016). Endothelial microparticles: Pathogenic or passive players in endothelial dysfunction in autoimmune rheumatic diseases? Vascul. Pharmacol. 86, 71-76. 10.1016/j.vph.2016.05.016 [DOI] [PubMed] [Google Scholar]
- Moroishi T., Hayashi T., Pan W.-W., Fujita Y., Holt M. V., Qin J., Carson D. A. and Guan K.-L. (2016). The Hippo pathway kinases LATS1/2 suppress cancer immunity. Cell 167, 1525-1539.e17. 10.1016/j.cell.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan J. F., Hu R., Oh R. S., Cohen S. N. and Lu Q. (2012). Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. USA 109, 4146-4151. 10.1073/pnas.1200448109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Tang K. D., Kenny L. and Punyadeera C. (2018). Salivary exosomes as potential biomarkers in cancer. Oral Oncol. 84, 31-40. 10.1016/j.oraloncology.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Neves R. F. C., Fernandes A. C. S., Meyer-Fernandes J. R. and Souto-Padrón T. (2014). Trypanosoma cruzi-secreted vesicles have acid and alkaline phosphatase activities capable of increasing parasite adhesion and infection. Parasitol. Res. 113, 2961-2972. 10.1007/s00436-014-3958-x [DOI] [PubMed] [Google Scholar]
- Nogueira P. M., Ribeiro K., Silveira A. C. O., Campos J. H., Martins-Filho O. A., Bela S. R., Campos M. A., Pessoa N. L., Colli W., Alves M. J. M. et al. (2015). Vesicles from different Trypanosoma cruzi strains trigger differential innate and chronic immune responses. J. Extracell Vesicles 4, 28734 10.3402/jev.v4.28734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberholzer M., Lopez M. A., McLelland B. T. and Hill K. L. (2010). Social motility in african trypanosomes. PLoS Pathog. 6, e1000739 10.1371/journal.ppat.1000739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., Chavez A. S. O., Felsheim R. F., Kurtti T. J. and Munderloh U. G. (2015). An Ixodes scapularis cell line with a predominantly neuron-like phenotype. Exp. Appl. Acarol. 66, 427-442. 10.1007/s10493-015-9908-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M., Carmo N. B., Krumeich S., Fanget I., Raposo G., Savina A., Moita C. F., Schauer K., Hume A. N., Freitas R. P. et al. (2010). Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 12, 19-30. 10.1038/ncb2000 [DOI] [PubMed] [Google Scholar]
- Raab-Traub N. and Dittmer D. P. (2017). Viral effects on the content and function of extracellular vesicles. Nat. Rev. Microbiol. 15, 559-572. 10.1038/nrmicro.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev-Rudzki N., Wilson D. W., Carvalho T. G., Sisquella X., Coleman B. M., Rug M., Bursac D., Angrisano F., Gee M., Hill A. F. et al. (2013). Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 153, 1120-1133. 10.1016/j.cell.2013.04.029 [DOI] [PubMed] [Google Scholar]
- Ribeiro K. S., Vasconcellos C. I., Soares R. P., Mendes M. T., Ellis C. C., Aguilera-Flores M., de Almeida I. C., Schenkman S., Iwai L. K. and Torrecilhas A. C. (2018). Proteomic analysis reveals different composition of extracellular vesicles released by two Trypanosoma cruzi strains associated with their distinct interaction with host cells. J. Extracell Vesicles 7, 1463779 10.1080/20013078.2018.1463779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu R., Kaushik S., Clement C. C., Cannizzo E. S., Scharf B., Follenzi A., Potolicchio I., Nieves E., Cuervo A. M. and Santambrogio L. (2011). Microautophagy of cytosolic proteins by late endosomes. Dev. Cell 20, 131-139. 10.1016/j.devcel.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio N. G., Emery S. J., Garnham A. L., Tan Q. Y., Sisquella X., Pimentel M. A., Jex A. R., Regev-Rudzki N., Schofield L. and Eriksson E. M. (2018). Extracellular vesicles from early stage Plasmodium falciparum-infected red blood cells contain PfEMP1 and induce transcriptional changes in human monocytes. Cell. Microbiol. 20, e12822 10.1111/cmi.12822 [DOI] [PubMed] [Google Scholar]
- Santangelo L., Giurato G., Cicchini C., Montaldo C., Mancone C., Tarallo R., Battistelli C., Alonzi T., Weisz A. and Tripodi M. (2016). The RNA-binding protein SYNCRIP is a component of the hepatocyte exosomal machinery controlling microRNA sorting. Cell Rep. 17, 799-808. 10.1016/j.celrep.2016.09.031 [DOI] [PubMed] [Google Scholar]
- Schorey J. S. and Harding C. V. (2016). Extracellular vesicles and infectious diseases: new complexity to an old story. J. Clin. Invest. 126, 1181-1189. 10.1172/JCI81132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorey J. S., Cheng Y., Singh P. P. and Smith V. L. (2015). Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 16, 24-43. 10.15252/embr.201439363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick A. E. and D'Souza-Schorey C. (2018). The biology of extracellular microvesicles. Traffic 19, 319-327. 10.1111/tra.12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. K., Kotsyfakis M. and Pedra J. H. F. (2016). For whom the bell tolls (and Nods): spit-acular saliva. Curr. Trop. Med. Rep. 3, 40-50. 10.1007/s40475-016-0072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff M. J., Temoche-Diaz M. M., Karfilis K. V., Ri S. and Schekman R. (2016). Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. eLife 5, 19276 10.7554/eLife.19276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J. M., Clos J., de'Oliveira C. C., Shirvani O., Fang Y., Wang C., Foster L. J. and Reiner N. E. (2010a). An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J. Cell Sci. 123, 842-852. 10.1242/jcs.056465 [DOI] [PubMed] [Google Scholar]
- Silverman J. M., Clos J., Horakova E., Wang A. Y., Wiesgigl M., Kelly I., Lynn M. A., McMaster W. R., Foster L. J., Levings M. K. et al. (2010b). Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J. Immunol. 185, 5011-5022. 10.4049/jimmunol.1000541 [DOI] [PubMed] [Google Scholar]
- Simbari F., McCaskill J., Coakley G., Millar M., Maizels R. M., Fabriás G., Casas J. and Buck A. H. (2016). Plasmalogen enrichment in exosomes secreted by a nematode parasite versus those derived from its mouse host: implications for exosome stability and biology. J. Extracell Vesicles 5, 30741 10.3402/jev.v5.30741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimo L., Kazimirova M., Richardson J. and Bonnet S. I. (2017). The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front. Cell Infect. Microbiol. 7, 281 10.3389/fcimb.2017.00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P. P., LeMaire C., Tan J. C., Zeng E. and Schorey J. S. (2011). Exosomes released from M. tuberculosis infected cells can suppress IFN-γ mediated activation of naïve macrophages. PLoS ONE 6, e18564 10.1371/journal.pone.0018564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisquella X., Ofir-Birin Y., Pimentel M. A., Cheng L., Abou Karam P., Sampaio N. G., Penington J. S., Connolly D., Giladi T., Scicluna B. J. et al. (2017). Malaria parasite DNA-harbouring vesicles activate cytosolic immune sensors. Nat. Commun. 8, 1985 10.1038/s41467-017-02083-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V. L., Cheng Y., Bryant B. R. and Schorey J. S. (2017). Exosomes function in antigen presentation during an in vivo Mycobacterium tuberculosis infection. Sci. Rep. 7, 43578 10.1038/srep43578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szempruch A. J., Sykes S.E., Kieft R., Dennison L., Becker A. C., Gartrell A., Martin W. J., Nakayasu E. S., Almeida I. C., Hajduk S. L. and Harrington J. M. (2016). Extracellular vesicles from Trypanosoma brucei mediate virulence factor transfer and cause host anemia. Cell 164, 246-257. 10.1016/j.cell.2015.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K., Tanaka N., Nakano T., Kakazu E., Kondo Y., Inoue J., Shiina M., Fukushima K., Hoshino T., Sano K. et al. (2010). Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem. Biophys. Res. Commun. 399, 384-390. 10.1016/j.bbrc.2010.07.083 [DOI] [PubMed] [Google Scholar]
- Teng G.-G., Wang W.-H., Dai Y., Wang S.-J., Chu Y.-X. and Li J. (2013). Let-7b is involved in the inflammation and immune responses associated with Helicobacter pylori infection by targeting Toll-like receptor 4. PLoS ONE 8, e56709 10.1371/journal.pone.0056709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C., Ostrowski M. and Segura E. (2009). Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581-593. 10.1038/nri2567 [DOI] [PubMed] [Google Scholar]
- Tirloni L., Reck J., Terra R. M. S., Martins J. R., Mulenga A., Sherman N. E., Fox J. W., Yates J. R. III, Termignoni C., Pinto A. F. M. et al. (2014). Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: a comparison between partially and fully engorged females. PLoS ONE 9, e94831 10.1371/journal.pone.0094831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirloni L., Islam M. S., Kim T. K., Diedrich J. K., Yates J. R. III, Pinto A. F. M., Mulenga A., You M.-J. and Da Silva Vaz I. Jr (2015). Saliva from nymph and adult females of Haemaphysalis longicornis: a proteomic study. Parasit. Vectors 8, 338 10.1186/s13071-015-0918-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brugger B. and Simons M. (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244-1247. 10.1126/science.1153124 [DOI] [PubMed] [Google Scholar]
- Trocoli Torrecilhas A. C., Tonelli R. R., Pavanelli W. R., da Silva J. S., Schumacher R. I., de Souza W., Cunha e Silva N., de Almeida Abrahamsohn I., Colli W. and Manso Alves M. J. (2009). Trypanosoma cruzi: parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect. 11, 29-39. 10.1016/j.micinf.2008.10.003 [DOI] [PubMed] [Google Scholar]
- van Niel G., D'Angelo G. and Raposo G. (2018). Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213-228. 10.1038/nrm.2017.125 [DOI] [PubMed] [Google Scholar]
- Villarreal A. M., Adamson S. W., Browning R. E., Budachetri K., Sajid M. S. and Karim S. (2013). Molecular characterization and functional significance of the Vti family of SNARE proteins in tick salivary glands. Insect Biochem. Mol. Biol. 43, 483-493. 10.1016/j.ibmb.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C., Gutiérrez-Vázquez C., Sánchez-Cabo F., Pérez-Hernández D., Vázquez J., Martin-Cofreces N., Martinez-Herrera D. J., Pascual-Montano A., Mittelbrunn M. and Sánchez-Madrid F. (2013). Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 4, 2980 10.1038/ncomms3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora A., Zhou W., Londono-Renteria B., Woodson M., Sherman M. B., Colpitts T. M., Neelakanta G. and Sultana H. (2018). Arthropod EVs mediate dengue virus transmission through interaction with a tetraspanin domain containing glycoprotein Tsp29Fb. Proc. Natl. Acad. Sci. USA 115, E6604-E6613. 10.1073/pnas.1720125115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Xi J., Hao X., Deng W., Liu J., Wei C., Gao Y., Zhang L. and Wang H. (2017). Red blood cells release microparticles containing human argonaute 2 and miRNAs to target genes of Plasmodium falciparum. Emerg. Microbes Infect. 6, 1-11. 10.1038/emi.2017.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Wang D., Jin F., Bian Z., Li L., Liang H., Li M., Shi L., Pan C., Zhu D. et al. (2017). Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat. Commun. 8, 14041 10.1038/ncomms14041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2017). Vector-Borne Diseases. WHO. [Google Scholar]
- Willms E., Johansson H. J., Mäger I., Lee Y., Blomberg K. E. M., Sadik M., Alaarg A., Smith C. I. E., Lehtiö J., El Andaloussi S. et al. (2016). Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 6, 22519 10.1038/srep22519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie M. P. and Ramirez M. I. (2017). Microvesicles released during the interaction between Trypanosoma cruzi TcI and TcII strains and host blood cells inhibit complement system and increase the infectivity of metacyclic forms of host cells in a strain-independent process. Pathog. Dis. 75, ftx077 10.1093/femspd/ftx077 [DOI] [PubMed] [Google Scholar]
- Xu B., Fu Y., Liu Y., Agvanian S., Wirka R. C., Baum R., Zhou K., Shaw R. M. and Hong T. T. (2017). The ESCRT-III pathway facilitates cardiomyocyte release of cBIN1-containing microparticles. PLoS Biol. 15, e2002354 10.1371/journal.pbio.2002354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian M., Fraser L. M., Agbedanu P. N., Harischandra H., Moorhead A. R., Day T. A., Bartholomay L. C. and Kimber M. J. (2015). Release of Small RNA-containing Exosome-like vesicles from the human filarial parasite Brugia malayi. PLoS Negl. Trop. Dis. 9, e0004069 10.1371/journal.pntd.0004069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Freitas D., Kim H. S., Fabijanic K., Li Z., Chen H., Mark M. T., Molina H., Martin A. B., Bojmar L. et al. (2018). Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 20, 332-343. 10.1038/s41556-018-0040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Woodson M., Neupane B., Bai F., Sherman M. B., Choi K. H., Neelakanta G. and Sultana H. (2018). Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells. PLoS Pathog. 14, e1006764 10.1371/journal.ppat.1006764 [DOI] [PMC free article] [PubMed] [Google Scholar]