Fig. 4.
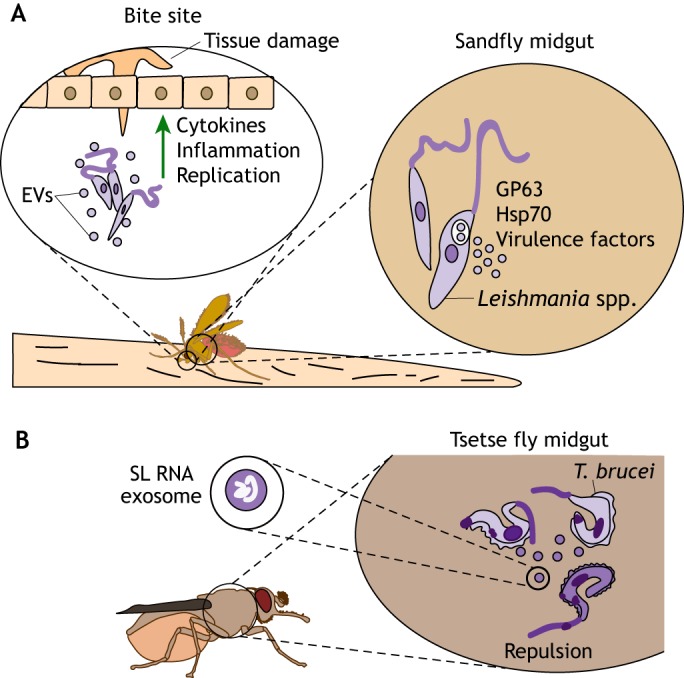
Parasite-derived extracellular vesicles influence vector–host interactions. (A) During replication within the sandfly midgut, Leishmania spp. secrete extracellular vesicles (EVs) that contain virulence factors and heat-shock proteins, such as GP63 and Hsp7. Blood-feeding sandflies inject these EVs into the bite site, along with the parasites, thereby stimulating the secretion of pro-inflammatory cytokines, increasing tissue damage and enhancing pathogen replication, which aggravates the disease. (B) EVs are involved in environmental sensing and signaling during replication within arthropod vectors. T. brucei uses EVs to signal stress conditions to other replicating parasites inside the midgut of tsetse flies. Unfit parasites (dark purple) secrete EVs containing SL RNA, which accumulates when mRNA trans-splicing is disrupted. These EVs are taken up by healthy parasites (light purple), leading to an arrest in their movement and/or change in direction. Intercommunication through EVs may enable successful vector colonization by these parasites to avoid an environment that is unfit for replication.
