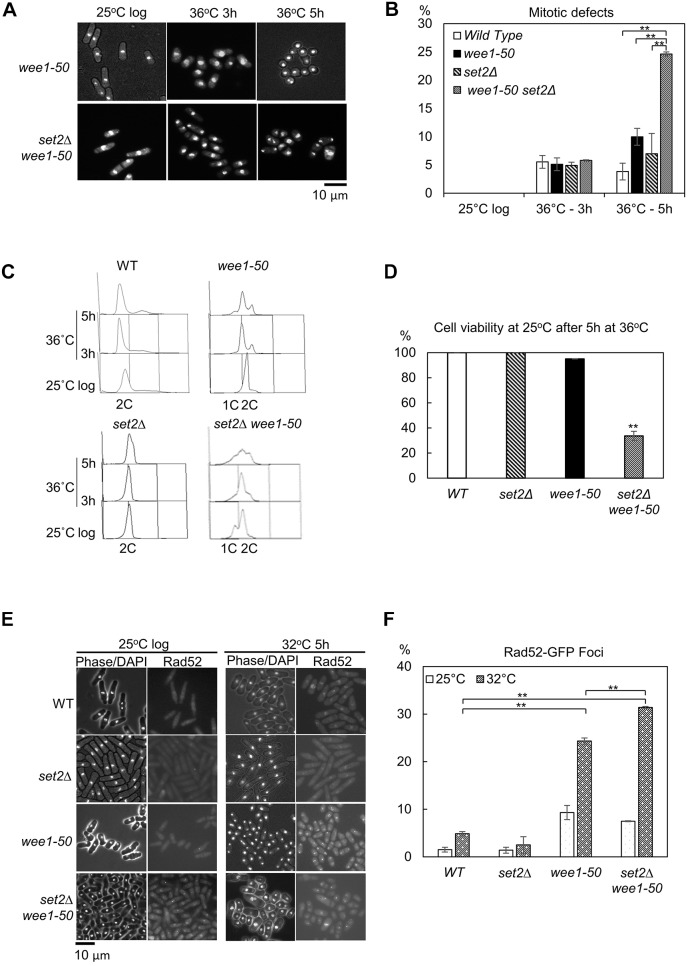
Fig. 4.
set2Δ synthetic lethality with wee1-50 results from replication catastrophe. (A) Inactivation of Wee1 in set2Δ cells results in premature entry into mitosis. wee1-50 or set2Δ wee1-50 cells were grown to log phase at the permissive temperature (25°C), then incubated at 36°C to inactivate Wee1. Samples were fixed with 70% ethanol at the indicated times. The fixed cells were stained with DAPI and examined by microscopy analysis. (B) Quantitative analysis of the percentage of cells with mitotic defect cells from experiments as in A. The data presented are from at least two independent biological repeats. **P<0.01 (t-test, n≥2 experiments for each genotype, n>200 cells for each data point). (C) WT, wee1-50, set2Δ and set2Δ wee1-50 strains were grown to log phase at the permissive temperature (25°C) then transferred to 36°C for the times shown. At the indicated times, cells were processed for FACS analysis. (D) WT, set2Δ, wee1-50 and set2Δ wee1-50 cells from the 5 h time point in (C) were collected, plated on the YES medium and incubated at 25°C for 3–4 days for viability analysis. (n≥2 experiments for each genotype, n>500 cells for each data point). **P<0.01 (t-test, between WT and set2Δ wee1-50 cells; P-value=0.0031). (E) Examination of Rad52–GFP foci in WT, set2Δ, wee1-50 and set2Δ wee1-50 cells at 25°C or 32°C. Cells were grown to log phase at the permissive temperature before being transferred to the semi-permissive temperature (32°C) for 5 h. Samples were fixed directly in methanol/acetone and examined by fluorescence microscopy. (F) The percentage of cells containing Rad52–GFP foci in the indicated strains is shown. **P<0.01 (t-test; n≥2 experiments, n≥100 cells for each data point). All error bars are s.e.m.