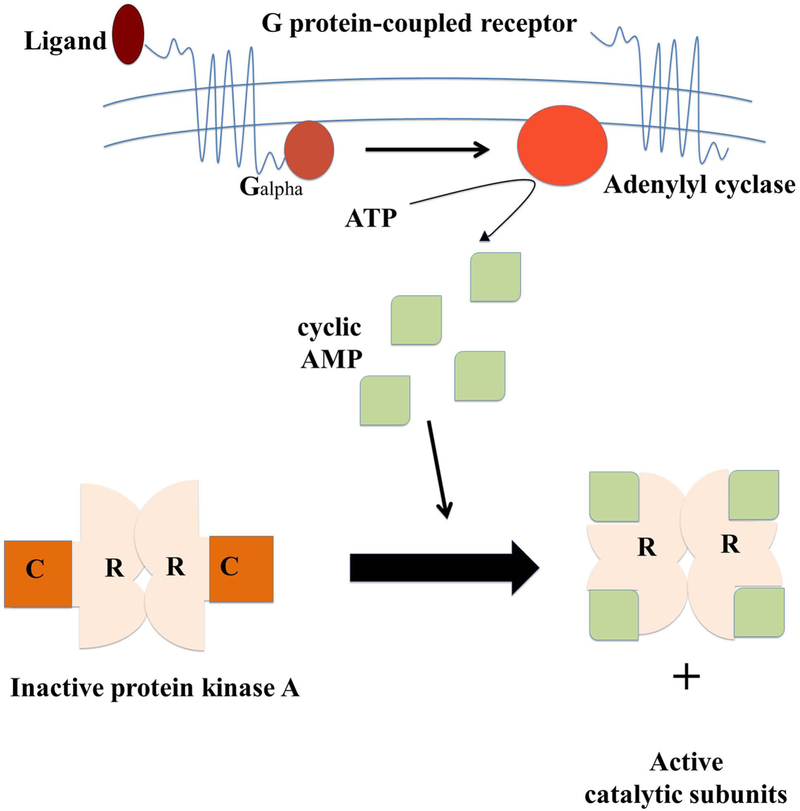
Figure 1.
Activation and inactivation mechanism of cAMP-dependent protein kinase A (PKA). When a G protein-coupled receptor is activated by its extracellular ligand, a conformational change is induced in the receptor, catalyzes the conversion of ATP into cyclic adenosine monophosphate (cAMP) increasing cAMP levels. Four cAMP molecules are required to activate a single PKA enzyme. This is done by two cAMP molecules binding to each of the two regulatory subunits (R) on a PKA enzyme causing the subunits to detach exposing the two (now activated) catalytic subunits (C). Next the catalytic subunits can go on to phosphorylate other proteins.