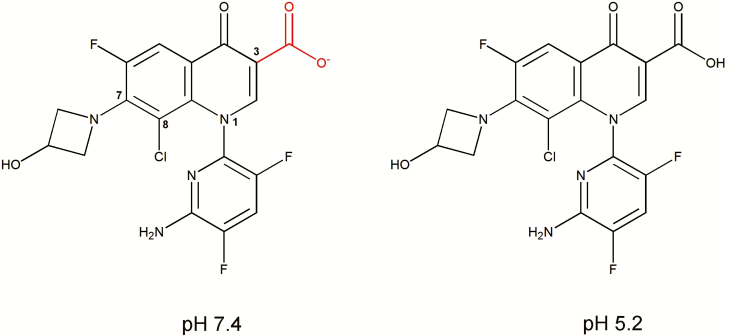
Figure 1.
Chemical structure of delafloxacin with atom numbering for the key positions discussed in the text. Due to the lack of basic group in the C7 substituent, the only ionizable group is the carboxylate function attached to position C3 (calculated pKa = 5.43). The figure shows the calculated predominant forms at pH 7.4 (left; anionic [to 98.5%]) and at pH 5.2 (right; neutral [62.7%]). Calculations were made with MarvinSketch version 18.9.0 (academic license) available from http://www.chemaxon.com.