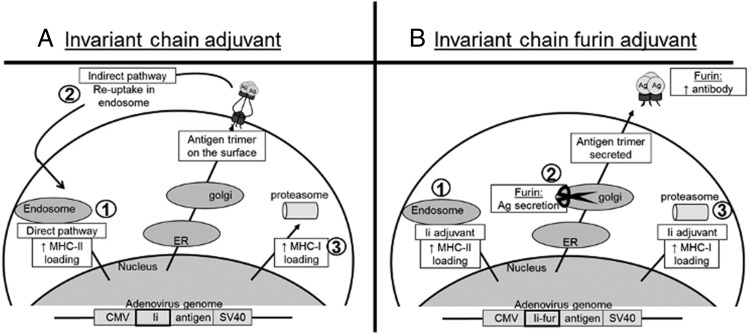
FIGURE 1.
Schematic theories of the intracellular mechanism and function of Ii and Ii-fur. (A) 1) After adenoviral infection, the Ii–Ag trimer (because of the Ii trimerization domain) is either transported to the endosomes through the ESS (1–17 first peptides), where the Ii–Ag complex will be degraded due to low pH. The presence of the complex in the endosomes increases the chances of the fused Ag peptides to be presented on MHCII and, therefore, enhances the CD4+ response to the Ag. This is called direct presentation. 2) The indirect presentation is due to the fact that Ii–Ag complex is also sorted to the TGN and will be presented on the surface of the cell, where it will be available for reuptake in the endosomes thanks to the ESS and follow the same route as the direct pathway from there. 3) Ii also increases MHCI presentation; however, the mechanism is not yet known. (B) After infection of the cells with the adenovirus inserted with Ii-fur adjuvant, as seen with the Ii, it can be transported either directly to the endosomes or through the TGN. 1) If it is directly transported to the endosomes, the direct pathway for increased MHCII should be conserved. 2) However, when the Ii is transported to the Golgi, the furin protease will be active and cleave the fur inserted in the complex prior to the trimerization domain. A cleaved protein in the Golgi will be sorted to the plasma membrane for secretion, and therefore a trimerized Ag fused to a part of Ii will be transported to the extracellular compartment to be secreted out of the cell. This will increase the accessibility of our Ag to both CD4+ T cells and B cells while retaining partly MHCII loading enhancement through the direct pathway and 3) similar to the Ii adjuvant, potential MHCI loading enhancement.