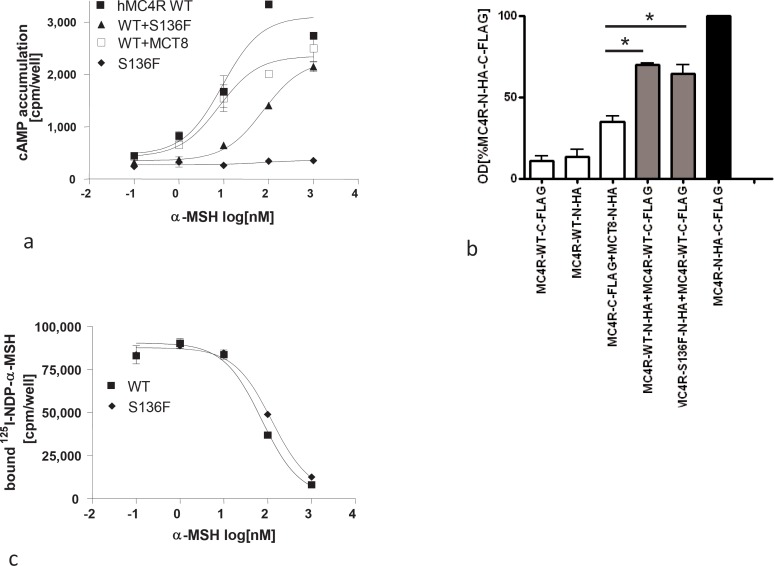
Fig. 1.
Functional characteristics of the MC4R S136F mutant: Assays were performed 48 h after transfection of WT and mutant constructs into COS7 cells. a cAMP accumulation after stimulation with different concentrations of α-MSH. Co-transfection of WT MC4R and thyroid hormone transporter MCT8 served as negative control. One representative experiment out of four performed in duplicates is shown. b Binding of 125I-NDP-α-MSH and displacement by α-MSH. Bmax of 125I-NDP-α-MSH at the WT MC4R was 91874.5 ± 6718 and at S136F mutant 87839 ± 3809 cpm. Displacement with unlabeled α-MSH resulted in IC50 values of 70.3 ± 11.64 and 125.7 ± 21.07 of WT and mutant MC4R, respectively. IC50 values were obtained using GraphPadPrism software. c Investigation of dimerization of the mutant S136F by a sandwich-ELISA approach. COS-7 cells were co-transfected with differentially tagged MC4R constructs, solubilized overnight and incubated in FLAG-antibody-coated 96-well plates. Dimerization was measured via the N-terminally tagged HA epitope as increase in optical density. WT-MC4R-CFL, WT-MC4R-NHA and co-transfected with WT-MC4R-C-FLAG with MCT8-N-HA served as negative controls, double tagged MC4R and co-transfection of WT-MC4R-N-HA and WT-MC4R-C-FLAG were used as positive controls. The mean absorption (492 nm / 620 nm) is shown as percentage of the double tagged WT-MC4R of three independent experiments. One way ANOVA and Tukey tests were conducted for the difference of the negative control (WT-MC4R-CFL+WT-MCT8-NHA) and two constructs (p < 0.001).