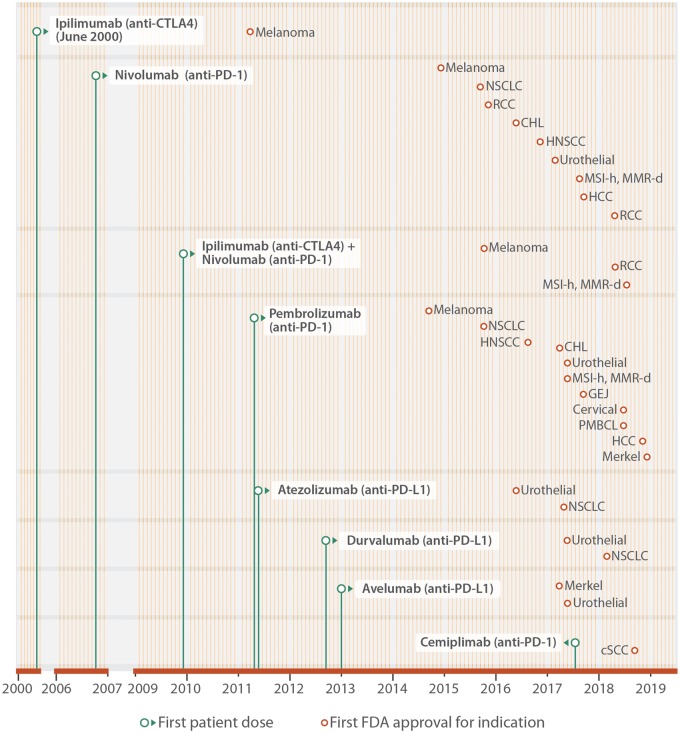
Figure 2.
Approved ICIs and indications. Monoclonal antibodies against CTLA-4 (ipilimumab), PD-1 (nivolumab, pembrolizumab, and cemiplimab) and PD-L1 (atezolizumab, avelumab, and durvalumab) have been approved by the FDA to treat patients with a variety of advanced and metastatic cancers. The period of clinical development is illustrated from the date of the first patient dosed (green dot), and until FDA approval for specific indications (brown dot). Each month of the year is represented by a vertical line (yellow). This figure extends earlier work by Ribas and Wolchok2 with indications approved by the FDA in 2018. RCC, renal cell carcinoma; MSI-H, high microsatellite instability; MMR-D, mismatch repair deficient; NSCLC, non-small cell lung cancer; CHL, classical Hodgkin’s lymphoma; HNC, head and neck cancer; CRC, colorectal cancer; HCC, hepatocellular carcinoma; GEJ, gastroesophageal junction; PMBCL, primary mediastinal large B cell lymphoma; SqCC, squamous cell carcinoma.