Figure 5.
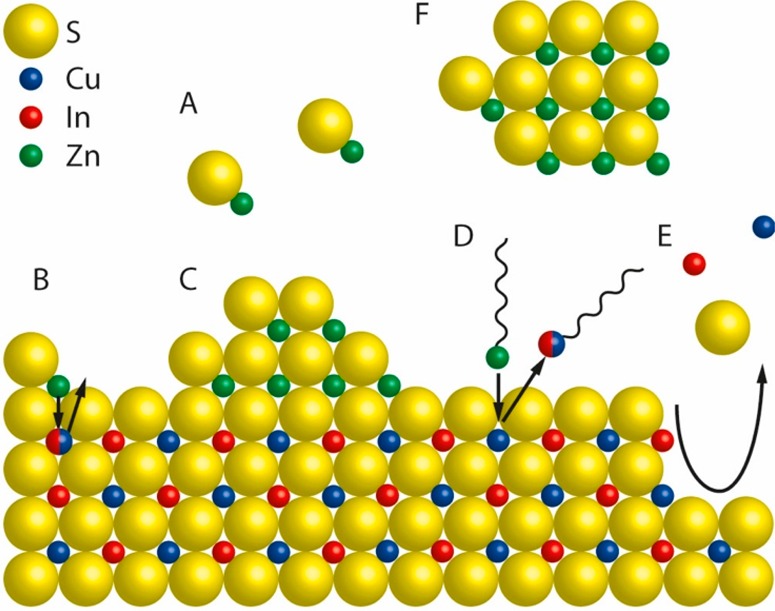
Schematic representation of a nanocrystal surface depicting the chemical processes that can take place during a shelling reaction, taking CuInS2/ZnS as an example. (A) [ZnS] monomers form homogeneously in solution from Zn- and S-precursors. (B) Alloying. Zn2+ from adsorbed [ZnS] monomer units diffuses inward while Cu+ and/or In3+ ions diffuse outward. (C) Heteroepitaxial shell overgrowth. A stable ZnS phase grows on the CuInS2 surface. (D) Cation exchange. Upon adsorption of Zn-R species (R= organic ligand) at the CuInS2 surface, a place-exchange reaction can occur, through which Zn2+ is incorporated in the NC, while Cu+ or In3+ cations are extracted as M-R species. (E) Etching. Chemical species in the reaction medium can promote the partial dissolution of the CuInS2 NC by extracting cations and/or S2– from the lattice. (F) Homogeneous nucleation. [ZnS] monomers can form ZnS NCs through homogeneous nucleation. Reproduced from ref (60). Copyright 2018 American Chemical Society.
