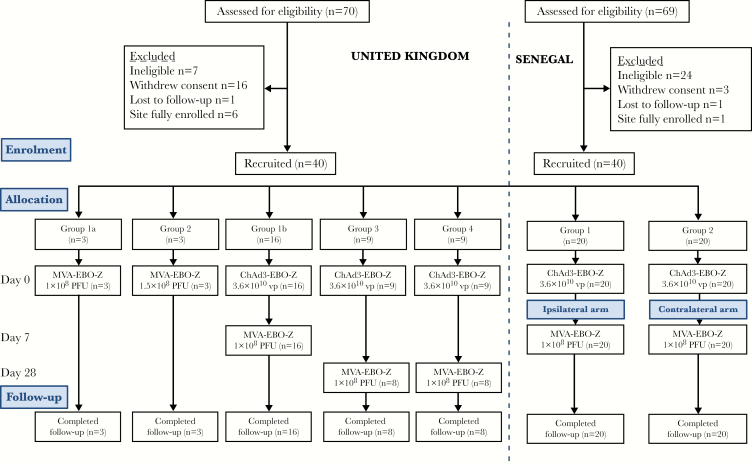
Figure 1.
Flowchart of study design and volunteer recruitment: Consolidated Standards of Reporting Trials (CONSORT) diagram of screening, enrollment, vaccination, and follow-up. All vaccinations were given intramuscularly. One volunteer in group 3 and 1 volunteer in group 4 withdrew from the UK study and were replaced; hence, n = 9 were allocated, but only 8 completed follow-up. This was unrelated to vaccination. All volunteers completed the study. There were no withdrawals in the Senegalese study and all volunteers completed the study. Abbreviations: ChAd3-EBO-Z, recombinant chimpanzee adenovirus type 3 vectored Ebola Zaire vaccine; MVA-EBO-Z, modified vaccinia Ankara virus vectored Ebola Zaire vaccine; pfu, plaque-forming units; vp, viral particles.