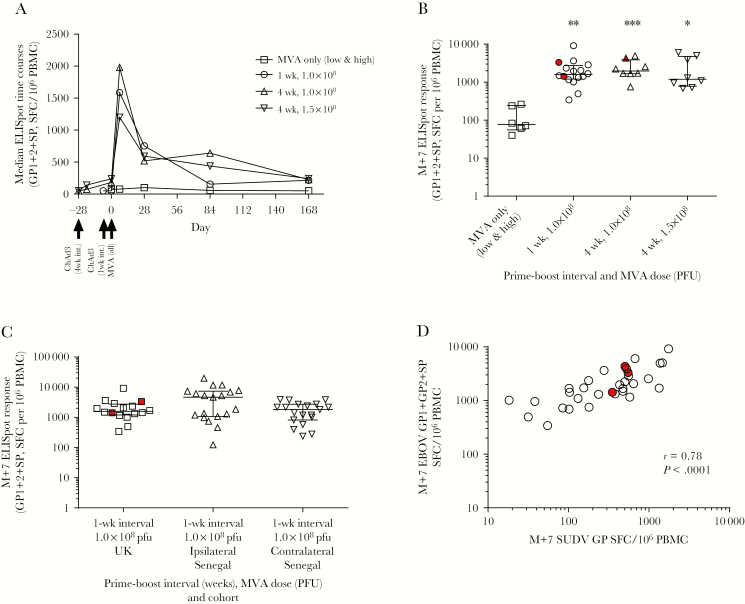
Figure 3.
Enzyme-linked immunospot assay (ELISpot) responses to vaccination. A, Median time courses of T-cell responses to vaccination in all UK volunteers. B, T-cell responses in each UK group at 1 week after modified vaccinia Ankara (MVA) (M+7) (Kruskal–Wallis test, P = .0014). C, Comparison of peak post-MVA responses (M+7) in Senegalese volunteers vaccinated with recombinant chimpanzee adenovirus type 3 vectored Ebola Zaire vaccine and boosted 1 week later with modified vaccinia Ankara virus vectored Ebola Zaire vaccine in either an ipsilateral or contralateral regimen compared with the dose- and interval-matched regimen in the United Kingdom. Kruskal–Wallis analysis with Dunn posttest, P = .237; Mann-Whitney test between ipsilateral and contralateral groups in Senegal, P = .0785. Only 3 volunteers across all UK groups received vaccines in a contralateral regimen (highlighted in red); all others received vaccines in an ipsilateral regimen. D, Association between the ELISpot responses to Sudan Ebola virus glycoprotein (GP) peptides and summed GP pools for Zaire Ebola virus in prime-boosted UK volunteers at M+7. Spearman r = 0.78, P < .0001. Black bars on column graphs indicate median and interquartile range. Abbreviations: ChAd3, chimpanzee adenovirus type 3; EBOV, Zaire Ebola virus; ELISpot, enzyme-linked immunospot assay; GP, glycoprotein; M+7, 7 days post– modified vaccinia Ankara; MVA, modified vaccinia Ankara; PFU, plaque-forming units; PBMC, peripheral blood mononuclear cells; SFC, spot-forming cells; SP, signal peptide; SUDV, Sudan Ebola virus; UK, United Kingdom.